
Concept explainers
One of the steps in the pentose phosphate pathway for glucose catabolism is the reaction of sedoheptulose 7-phosphate with glyceraldehydes 3-phosphate in the presence of a transaldolase to yield erythrose 4-phosphate and fructose 6-phosphate.
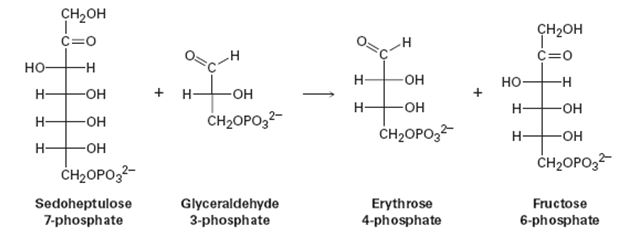
(a) The first part of the reaction is the formation of a protonated Schiff base of sedoheptulose 7-phosphate with a lysine residue in the enzyme followed by a retro-aldol cleavage to give an enamine plus erythrose 4-phosphate. Show the structure of the enamine and the mechanism by which it is formed.
(b) The second part of the reaction is a nucleophilic addition of the enamine to glyceraldehyde 3-phosphate followed by hydrolysis of the Schiff base to give fructose 6-phosphate. Show the mechanism.
Trending nowThis is a popular solution!

Chapter 29 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Basic Chemistry (5th Edition)
Organic Chemistry
Chemistry: Structure and Properties
General, Organic, & Biological Chemistry
Organic Chemistry
Chemistry & Chemical Reactivity
- One of the steps in the pentose phosphate pathway for glucose catabolism is the reaction of xylulose 5-phosphate with ribose 5-phosphate in the presence of a transketolase to give glyceraldehyde 3-phosphate and sedoheptulose 7-phosphate. (a) The first part of the reaction is nucleophilic addition of thiamin diphosphate (TPP) ylide to xylulose 5-phosphate, followed by a retro-aldol cleavage to give glyceraldehyde 3-phosphate and a TPPcontaining enamine. Show the structure of the enamine and the mechanism by which it is formed. (b) The second part of the reaction is addition of the enamine to ribose 5-phosphate followed by loss of TPP ylide to give sedoheptulose 7-phosphate. Show the mechanism.arrow_forwardIn glycerol metabolism, the oxidation of sn-glycerol 3-phosphate to give dihydroxyacetone phosphate is catalyzed by sn-glycerol-3-phosphate dehydrogenase, with NAD+ as cofactor. The reaction is stereospecific, occurring exclusively on the Re face of the nicotinamide ring.arrow_forwardThe dehydration of citrate to yield cis-aconitate, a step in the citric acid cycle, involves the pro-R “arm’’ of citrate rather than the pro-S arm. Which of the following two products is formed?arrow_forward
- In one of the steps in this pathway fructose 1,6-biphosphate (F1,6BP) is converted to gylceraldehyde-3-phosphate (G-3-p) and dihydroxyacetone phosphate (DHAP). This reaction is catalyzed by the enzyme aldolase. For this reaction at 25 celcuius and Ph7 we have: Keq= 10-4M and Delta G = +5456 cal/mol Calculate the following: The concentration of F1, 6BP, DHAP and G-3-P at equilibrium when the initial F1,6BP is (A) 1M, (b) 10-2 M, (c) 2 X 10*4 M and (d) 10-5 M. I been trying to solve this problem but I dont even know where to begin with. Atleast help me with problem A, I would be able to guide myself through.arrow_forward3) The enzyme aldolase catalyzes the conversion of fructose-1,6-diphosphate (FDP)to dihydroxyacetone phosphate (DHAP) and glyceraldehide-3-phosphate (G3P). Thereaction isFDP ⇄ DHAP + G3P , ∆rG0(298.15 K) = 23.8 kJ mol−1In red blood cells the concentration of these species are [FDP] = 35 µM, [DHAP] =130 µM, and [G3P] = 15 µM. (Remember that 1.0 µM = 1.0 × 10−6 mol L−1. Thestandard state for reactions in solution can be taken as c0 = 1.0 mol L−1).Calculate ∆rG in a red blood cell at 25 0C. Will the reaction occur spontaneouslyin the cell at this temperature?arrow_forwardAldolase catalyzes the glycolytic reaction The standard free-energy change for this reaction in the direction written is +23.8 kJ/mol. The concentrations of the three intermediates in the hepatocyte of a mammal are: fructose 1,6-bisphosphate, 1.4 × 10−5 M; glyceraldehyde 3-phosphate, 3 × 10−6 M; and dihydroxyacetone phosphate, 1.6 ×10−5 M. At body temperature (37 °C), what is the actual free-energy change for the reaction?arrow_forward
- Triosephosphate isomerase (TIM) catalyzes the conversion of dihydroxyacetone phosphate to glyceraldehyde-3-phosphate. The enzyme’s catalytic groups are Glu 165 and His 95. In the first step of the reaction, these catalytic groups function as a general-base and a general-acid catalyst, respectively. Propose a mechanism for the reaction.arrow_forward2) True or False: C5a catalyzes production of C3b.arrow_forwardThe tartaness of some wines is high concentration of l malate.write a consequence of reactions showing how yeast cells synthesize l malate from glucose under anaerobic conditions in the presence of co2arrow_forward
- Which of the reactions are spontaneous (favorable)? 1)DHAP↽−−⇀glyceraldehyde-3-phosphateΔ?=3.8 kJ/mol 2)L-malate+NAD+⟶oxaloacetate+NADH+H+Δ?=29.7 kJ/mol- 3) glutamate+NAD++H2O⟶NH+4+α-ketoglutarate+NADH+H+Δ?=3.7 kcal/ 4) C6H13O9P+ATP⟶C6H14O12P2+ADPΔ?=−14.2KJ/mol 5) −Rh(I)C2H6Δ?=−150.97 kJ/molC2H4+H2→Rh(I)C2H6ΔG=−150.97 kJ/mol 6) C4H4O5⟶C4H2O4+H2OΔ?=3.1 kJ/molarrow_forwardIn the pentose phosphate pathway for degrading sugars, ribulose 5-phosphate is converted to ribose 5-phosphate. Propose a mechanism for the isomerization.arrow_forwardImidazoleglycerol‑phosphate dehydratase is an enzyme in the histine biosynthesis pathway. It catalyzes the E1 dehydration of D‑erthyro‑imidazole‑glycerol phosphate to imidazole acetol‑phosphate. This is a rare example of a biological E1 reaction, as most biological elimination reactions occur through E1cB instead. In this reaction, D‑erthyro‑imidazole‑glycerol phosphate is first protonated to form a good leaving group. Then, the leaving group is ejected to form the resonance‑stabilized carbocation shown. Draw curved arrows forming the most stable resonance structure to explain why this reaction goes through an E1 mechanism. Draw curved arrows to form the most stable resonance structure.arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

