
(a)
Interpretation:
The condensed structural formula of
Concept introduction:
The condensed structural formula is a way of representation or writing organic structures in a line. The lines are drawn between bonded atoms, but the bond between carbon-hydrogen should be omitted.
Answer to Problem 18E
The condensed structural formula of
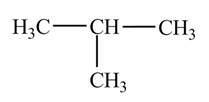
Explanation of Solution
The
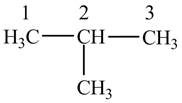
Figure 1
The numbering is assigned to the carbon atoms according to the longest continuous chain of carbon atoms. The
The condensed structural formula of
(b)
Interpretation:
The condensed structural formula of
Concept introduction:
The condensed structural formula is a way of representation or writing organic structures in a line. The lines are drawn between bonded atoms, but the bond between carbon-hydrogen should be omitted.
Answer to Problem 18E
The condensed structural formula of
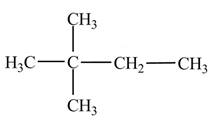
Explanation of Solution
The
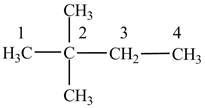
Figure 2
The numbering is assigned to the carbon atoms according to the longest continuous chain of carbon atoms. The
The condensed structural formula of
(c)
Interpretation:
The condensed structural formula of
Concept introduction:
The condensed structural formula is a way of representation or writing organic structures in a line. The lines are drawn between bonded atoms, but the bond between carbon-hydrogen should be omitted.
Answer to Problem 18E
The condensed structural formula of
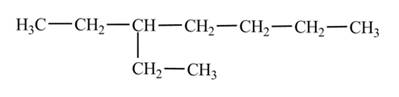
Explanation of Solution
The
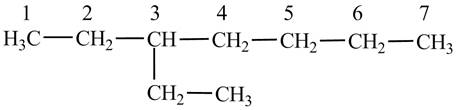
Figure 3
The numbering is assigned to the carbon atoms according to the longest continuous chain of carbon atoms. The
The condensed structural formula of
(d)
Interpretation:
The condensed structural formula of
Concept introduction:
The condensed structural formula is a way of representation or writing organic structures in a line. The lines are drawn between bonded atoms, but the bond between carbon-hydrogen should be omitted.
Answer to Problem 18E
The condensed structural formula of
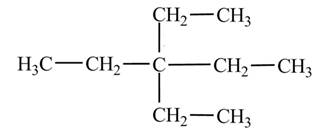
Explanation of Solution
The
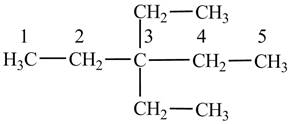
Figure 4
The numbering is assigned to the carbon atoms according to the longest continuous chain of carbon atoms. The diethyl represents that two ethyl
The condensed structural formula of
Want to see more full solutions like this?
Chapter 19 Solutions
INTRODUCTORY CHEMISTRY-STD.GDE.+SOL.MAN
- Given the line/skeletal structure, provide the IUPAC names of the following organic compounds. (Please refer to the picture below)arrow_forwardDraw the structure (Optional Condensed or Line) for the following Organic compounds. By using IUPAC system. A. 2,4-di ethoxy hexane B. 3-bromo-2-floro-3-methy hexanol C. 3,4-dimethyl pentan-2-one D. Fluoro 2-bromo-3-chloro pentanoate In the image, name the following organic molecules by using IUPAC rules.arrow_forwardWrite the IUPAC names of the following stuctures of organic compounds.arrow_forward
- What is the CONDENSED structural formula for 1,3-dichloropropanearrow_forwardWrite the condensed structural formula for butyl ethyl ether.arrow_forwardWhat is the IUPAC name for the compound shown? How do I differentiate between the ethyl groups and the methyl groups using only the skeletal structure?arrow_forward
