
Concept explainers
Identify the
a. tyrosine (neutral, polar)
b. glutamate (acidic, polar)
c. methionine (neutral, nonpolar)
d. histidine (basic, polar)
e. cysteine (neutral, polar)
f. valine (neutral, nonpolar)
(a)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
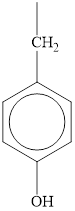
The structure of tyrosine is given below.
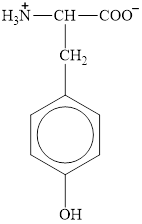
Figure 1
Therefore, the

Figure 2
The
(b)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
![]()
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
The structure of glutamate is given below.
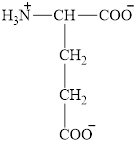
Figure 3
Therefore, the
![]()
Figure 4
The
(c)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
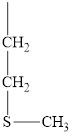
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
The structure of methionine is given below.
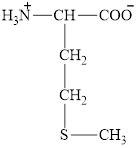
Figure 5
Therefore, the
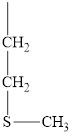
Figure 6
The
(d)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
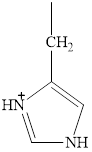
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
The structure of histidine is given below.
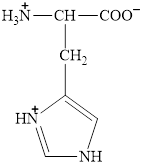
Figure 7
Therefore, the

Figure 8
The
(e)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
![]()
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
The structure of cysteine is given below.
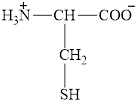
Figure 9
Therefore, the
![]()
Figure 10
The
(f)
Interpretation:
The
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while other elements are found in side chains.
Answer to Problem 19.3E
The
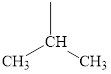
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore is known as carboxylate group.
The structure of valine is given below.
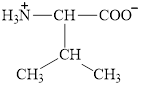
Figure 11
Therefore, the
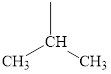
Figure 12
The
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- Glutathione (G-SH), one of the most common tripeptides in animals, plants, and bacteria, is a scavenger of oxidizing agents. In reacting with oxidizing agents, glutathione is converted to G-S-S-G. (a) Name the amino acids in this tripeptide. (b) What is unusual about the peptide bond formed by the N-terminal amino acid? (c) Write a balanced half-reaction for the reaction of two molecules of glutathione to form a disulfide bond. Is glutathione a biological oxidizing agent or a biological reducing agent? (d) Write a balanced equation for reaction of glutathione with molecular oxygen, O2 to form G-S-S-G and H2O. Is molecular oxygen oxidized or reduced in this process?arrow_forwardConsider the tripeptide tyrosylleucylisoleucine. a. Specify its structure using three-letter symbols for the amino acids. b. How many peptide bonds are present within the peptide? c. Which of the amino acid residues has the largest R group? d. Which of the amino acid residues, if any, has an acidic side chain?arrow_forwardConsider the tripeptide leucylvalyltryptophan. a. Specify its structure using three-letter symbols for the amino acids. b. How many peptide bonds are present within the peptide? c. Which of the amino acid residues has the largest R group? d. Which of the amino acid residues, if any, has a basic side chain?arrow_forward
- 22-30 (a) Use the three-letter abbreviations to write a representation of the following tripeptide: (b) Which amino acid is at the C-terminal end, and which is at the N-terminal end?arrow_forward22-42 (a) How many atoms of the peptide bond lie in the same plane? (b) Which atoms are they?arrow_forwardDrawing Peptide Structures Draw the tripeptide that would result from cysteine attaching to the previous dipeptide on the acidic side.arrow_forward
- Arginine exists in two high-pH forms instead of the usual one. Explain why.arrow_forwardWhat characteristics indicate that amino acids exist as zwitterions?arrow_forwardGive one example of a conjugated protein that contains the following prosthetic group: a.iron b.lipid c.phosphate group d.carbohydrate e.hemearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





