
Concept explainers
(a)
Interpretation:
The high-spin and low-spin complexes have to be identified and the number of unpaired electrons present in each complex has to be given from given set of complexes.
(a)
Explanation of Solution
For
Ground state electronic configuration of
Ground state electronic configuration of
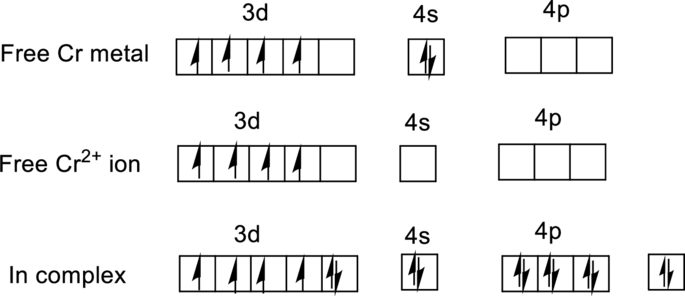
Water is a weak ligand so it is forms a high-spin complex with
For
Ground state electronic configuration of
Ground state electronic configuration of
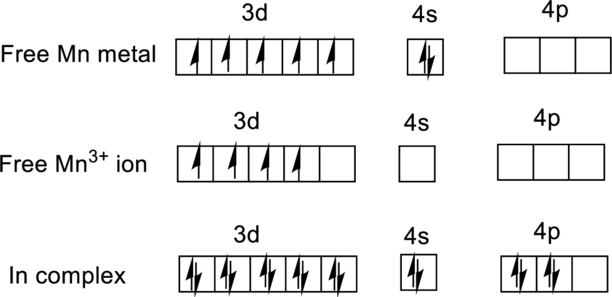
(b)
Interpretation:
The high-spin and low-spin complexes have to be identified and the number of unpaired electrons present in each complex has to be given from given set of complexes.
(b)
Explanation of Solution
For
Ground state electronic configuration of
Ground state electronic configuration of
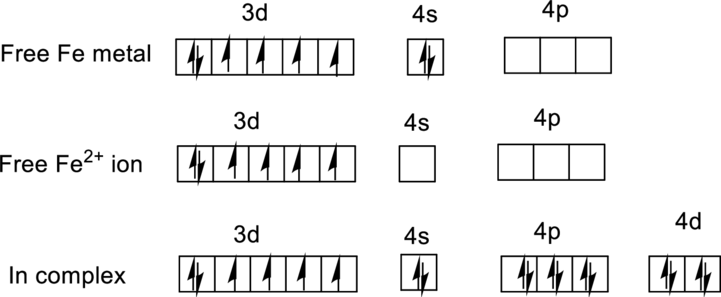
Water is a weak ligand so it is forms a high-spin complex with
For
Ground state electronic configuration of
Ground state electronic configuration of
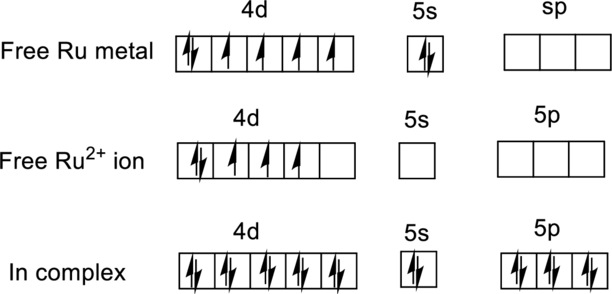
(c)
Interpretation:
The high-spin and low-spin complexes have to be identified and the number of unpaired electrons present in each complex has to be given from given set of complexes.
(c)
Explanation of Solution
For
Ground state electronic configuration of
Ground state electronic configuration of
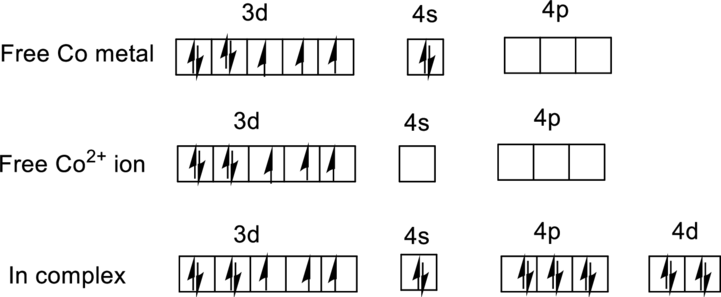
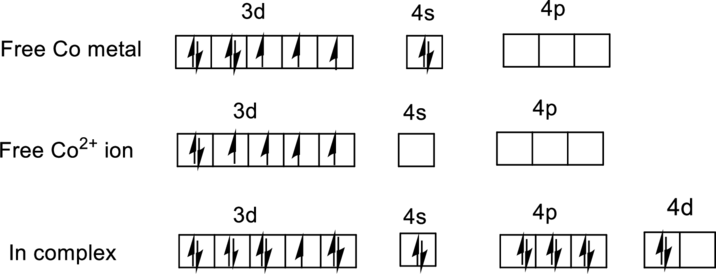
Water is a weak ligand so it is forms a high-spin complex with
For
Ground state electronic configuration of
Ground state electronic configuration of
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry: Principles and Practice
- Four different octahedral chromium coordination compounds exist that all have the same oxidation state for chromium and have H2O and Cl as the ligands and counterions. When 1 mole of each of the four compounds is dissolved in water, how many moles of silver chloride will precipitate upon addition of excess AgNO3?arrow_forwardFor each d electron configuration, state the number of unpaired electrons expected in octahedral complexes. Give an example complex for each case. (Two answers are possible for some of these cases.) (a) d2 (b) d4 (c) d6 (d) d8arrow_forwardPlatinum(II) forms many complexes, among them those with the following ligands. Give the formula and charge of each complex. (a) two ammonia molecules and one oxalate ion (C2O42-) (b) two ammonia molecules, one thiocyanate ion (SCN-), and one bromide ion (c) one ethylenediamine molecule and two nitrite ionsarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





