
Concept explainers
PRACTICE PROBLEM 19.1
(a) Write a mechanism. for all steps of the Claisen condensation that take place when ethyl propanoate reacts with ethoxide ion.
(b) What products form when the reaction mixture is acidified?
Interpretation: The mechanism for all the steps of Claisen condensation when ethyl propanate reacts with ethoxide ion is to be written and when the reaction mixture acidified the product of the reaction is to be determined.
Concept introduction:
The Claisen condensation is the carbon-carbon bond forming reaction and important for the preparing
In Claisen condensation ester of one molecule adds to the carbonyl carbon of the another molecule and formation of
Answer to Problem 1PP
Solution:
(a)
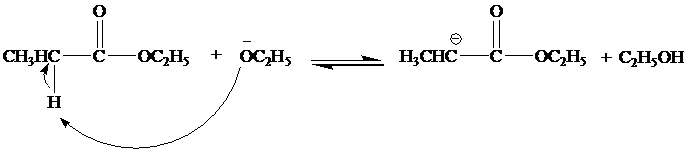
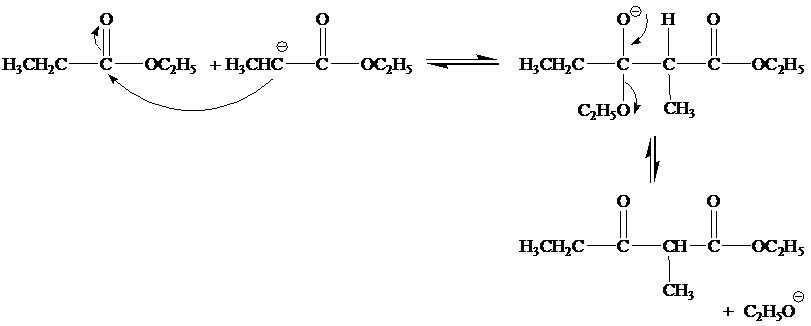

(b)
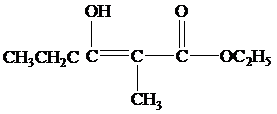
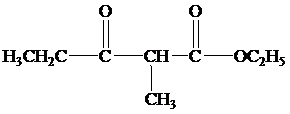
Explanation of Solution
The mechanism for the reaction between ethylproponate and ethoxide ion is as follows:
Step 1
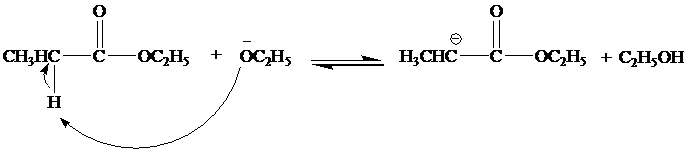
Step 2
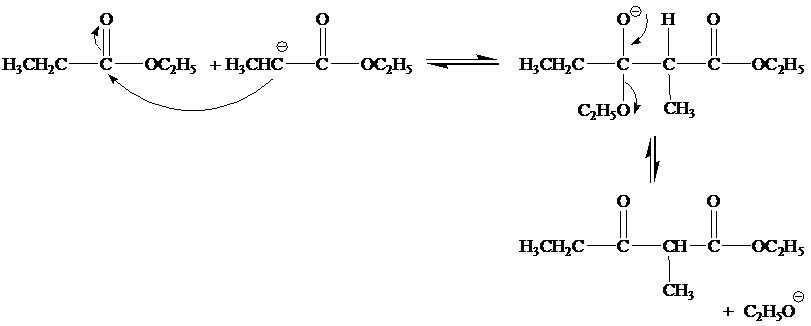
Step 3

(b)
When the reaction mixture is acidified the reaction takes place is as follows;
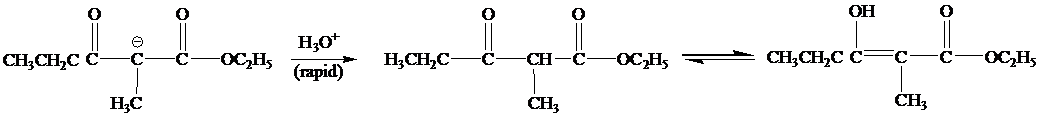
Hence, the product formed when the reaction mixture acidified is as follows;
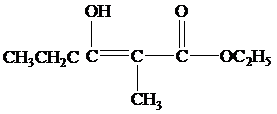
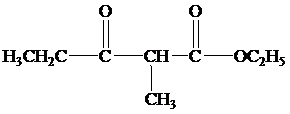

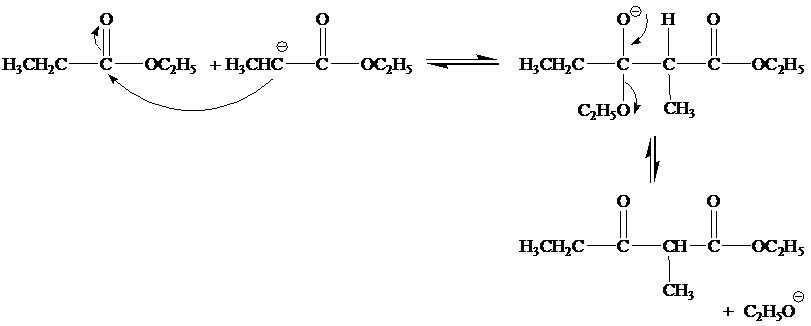

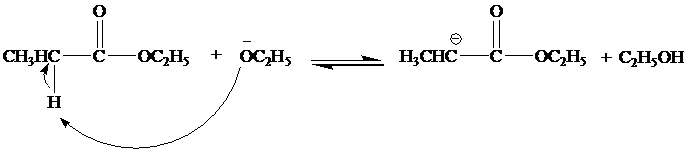
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry, 12e Binder Ready Version Study Guide / Student Solutions Manual
Additional Science Textbook Solutions
Principles of Chemistry: A Molecular Approach (3rd Edition)
Chemistry & Chemical Reactivity
General, Organic, and Biological Chemistry (3rd Edition)
Organic Chemistry (8th Edition)
Chemistry
Chemistry: The Molecular Nature of Matter
- Wolff-Kishner reduction of compound W gave compound A. Treatment of A with m-chloroperbenzoic acid gave B which on reduction with LiAH4 gave C. Oxidation of compound C with chromic acid gave D (C9H14O). Suggest the structures for A, B, C, and D.arrow_forwardA certain compound is known to contain an aromatic benzene ring but failed to produce a fragrant yellow solution upon subjecting it to the nitration test. What may be a possible explanation for this? A. The benzene ring is part of a highly conjugated, blue dye molecule. B. The benzene ring contains a strong electron-withdrawing group. C. The benzene ring has no available sites left for electrophilic attack. D. All of the given. Kindly explain your answer in detail.arrow_forwardSuggest reactivity of compound A, B and C in increasing order of E2 reactionarrow_forward
- When the nitrogen-containing aromatic heterocyclic compounds 1 and 2 are treated with HCl, only 1 forms the hydrochloride salt, whereas compound 2 is unreactive. Provide an explanation for this observed reactivity.arrow_forwardPropose a feasible synthesis for diphenylacetylene from bromobenzene. Draw the reaction scheme and briefly describe the reaction.arrow_forwardComplete the following reaction sequences by drawing the major products or the reagents necessary to make them. Be sure to include stereochemistry when appropriate. dont provide handwritten solutionarrow_forward
- Provide reagents and conditions for the following synthetic tranformation. (Please don't provide handwritten solution)arrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.)p-Chlorobenzoic acidarrow_forward(a) Illustrate the following name reactions by giving example :(i) Cannizzaro’s reaction(ii) Clemmensen reduction(b) An organic compound A contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollen’s reagent but forms an addition compound with sodium hydrogen sulphite and gives positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acids. Derive the possible structure of compound A.arrow_forward
- Suggest reasonable explanations for each of the following: A. The rate of saponification of ethyl p-nitrobenzoate is 200 times faster than that of ethyl p-methoxybenzoate. B. The rate of saponification of methyl acetate, CH3CCO2CH3 is 50 times greater than that for isopropylacetate.arrow_forwardTwo isomers, A and B, of molecular formula C5H8 undergo catalytic hydrogenation with hydrogen gas and palladium on carbon to form the same C5H10 product. On ozonolysis followed by treatment with hydrogen peroxide (H2O2), isomer A gave a product of molecular formula C5H8O4 that has two carboxylic acid groups in it whereas isomer B gave a product of molecular formula C5H8O3 that contains a carboxylic acid group and a ketone group. Which of the following isomeric pairs best match this data?arrow_forwardTwo isomers, A and B, of molecular formula C5H8 undergo catalytic hydrogenation with hydrogen gas and palladium on carbon to form the same C5H10 product. On ozonolysis followed by treatment with hydrogen peroxide (H2O2), isomer A gave a product of molecular formula C5H8O4 that has two carboxylic acid groups in it whereas isomer B gave a product of molecular formula C5H8O3 that contains a carboxylic acid group and a ketone group. What is the isometric pair of A and B that corresponds?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

