
Concept explainers
(a)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group,
Answer to Problem 44E
The class of compound
Explanation of Solution
The given organic compound is
Alcohol is the class of organic compounds in which an alkyl group is attached to hydroxyl group. The general formula of alcohols is
Therefore, the class of compound
The class of given has been rightfully identified.
(b)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 44E
The class of compound
Explanation of Solution
The given organic compound is
Ether is the derivatives of alcohol. The general formula of alcohols is shown below.
When the hydrogen of the alcohol is replaced by another alkyl group ether is formed. The general formula of ether is shown below.
Therefore, the class of compound
The class of given compound has been rightfully identified.
(c)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 44E
The class of the given compound is amide.
Explanation of Solution
The structure of the given compounds is shown below.
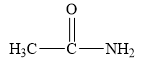
Figure 1
Amides are the derivatives of carboxylic acid. The general structure of carboxylic acid is shown below.
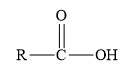
Figure 2
When the hydroxyl group of carboxylic acid is replaced by
Therefore, the class of the given compound is amide.
The class of given has been rightfully identified.
(d)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 44E
The class of the given compound is ketone.
Explanation of Solution
The structure of the given organic compound is shown below.
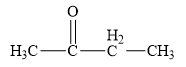
Figure 3
The carbonyl compounds are the compounds that contains
Therefore, the class of the given compound is ketone.
The class of given has been rightfully identified.
(e)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 44E
The class of the given compound is ester.
Explanation of Solution
The structure of the given compounds is shown below.
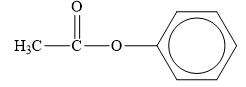
Figure 4
Esters are the derivatives of carboxylic acid. The general structure of carboxylic acid is shown below.
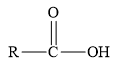
Figure 2
When the hydrogen of the hydroxyl group of carboxylic acid is replaced by an alkyl or aryl group, then ester is formed.
Therefore, the class of the given compound is ester.
The class of given has been rightfully identified.
(f)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 44E
The class of given compound is phenol.
Explanation of Solution
The structure of the given organic compound is shown below.
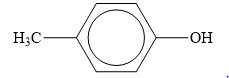
Figure 5
When an
Therefore, the class of given compound is phenol.
The class of given has been rightfully identified.
(g)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 44E
The class of the given compound is organic halide.
Explanation of Solution
The structure of the given organic compound is shown below.
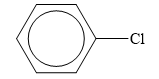
Figure 6
Halogen is the elements that belong to the seventeenth group of the periodic table. When a halogen is combined to a hydrocarbon, then organic halides are formed. Chlorine is halogen.
Therefore, the class of the given compound is organic halide.
The class of given has been rightfully identified.
(h)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 44E
The class of the given compound is alcohol.
Explanation of Solution
The structure of the given compounds is shown below.
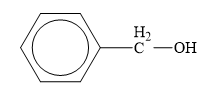
Figure 7
Alcohol is the class of organic compounds in which an alkyl group is attached to hydroxyl group. The general formula of alcohols is
Therefore, the class of the given compound is alcohol.
The class of given has been rightfully identified.
Want to see more full solutions like this?
Chapter 19 Solutions
INTRO CHEM + MASTERING W/ETEXT
- Which of the structural formulas represents methanoic acid?arrow_forwardWhat is the relationship of the two compounds in each of the following pairs?arrow_forwardIdentify the functional group in the following molecule. Your choices are alkene, alkyne, aromatic, alcohol, thiol, ether, aldehyde, ketone, carboxylic acid, ester, or amine.arrow_forward
- Which of the following is a furan?arrow_forwardDraw the condensed structures of the compounds formedfrom (a) butanoic acid and methanol, (b) benzoic acid and2-propanol, (c) propanoic acid and dimethylamine. Namethe compound in each case.arrow_forwardDetermine the molecular formula for each of the following.In other words, supply the subscripts.arrow_forward
- Write a condensed structural formula for an acid with the formula C4H8O2arrow_forwardFor each of the following, state whether the two structural formulae show representconstitutional isomers or same compounds or different compounds.arrow_forwardA peptide bond links two amino acids together. It is an amide bond because the atoms joined by this bond are _____, and RCONR'R" is the general form of an amide.arrow_forward
- Select the functional groups that are PRESENTS in the compund. Choose between these options below. -Ketone, Aromatic, Amine, Carboxylic Acid, Amide, Aldehyde, Ether, Ester.arrow_forwardChloropropane is derived from propane by substituting Clfor H on one of the carbon atoms. (a) Draw the structuralformulas for the two isomers of chloropropane. (b) Suggestnames for these two compounds.arrow_forwardWhat is the molecular formula of the compound shown?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





