
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.9, Problem DQ
The following structure is called imidazolium. Which of the following statements about imidazolium are true?
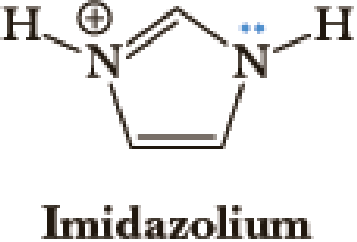
- a. Both nitrogens are sp2 hybridized, and the lone pair of electrons is in a 2p orbital.
- b. The nitrogen on the right is sp3 hybridized, while the nitrogen on the left is sp2 hybridized. The lone pair of electrons is in an sp3 hybrid orbital.
- c. The molecule has an equivalent contributing structure not shown.
- d. The molecule has no reasonable contributing structures.
- 1. Statements a and c are true.
- 2. Statements a and d are true.
- 3. Statements b and c are true.
- 4. Statements b and d are true.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Harry creates a compound that contains only 1 pi (π) bond in total. The central atom has 4s2, 3d10, and 4p5. There are only 3 surrounding atoms with the electron configuration: 1s2 2s2 2p5. What is the formula and VSEPR shape for this compound? Explain how this is possible with respect to hybridized orbitals and how electrons are moved around to create this compound.
Two isomers share the molecular formula C3H4. In Structure A two ofthe carbon atoms are sp hybridized and one carbon atom is sp3 hybridized. In Structure B, two of the carbon atoms are sp2 hybridized and one carbon atom is sphybridized.
b)Draw “3D”-looking structures on paper, make a sketch of each of the isomers. (This should be a sketch of themolecules, not diagrams of overlapping orbitals!) Your drawing should show thecorrect molecular geometry around each carbon atom. [HINT: NEITHER of thesemolecules is planar!!! To draw them correctly, you will have to consider why!]
What statement about sigma(lowercase) bonds is true?
a)They are created by the overlap of p orbitals
b)They only occur in single bonds
c)It's the first covalent bond between two atoms in a molecular compound
d)They only occur between hybridized orbitals
e)none on the above
Chapter 1 Solutions
Organic Chemistry
Ch. 1.1 - Prob. 1.1PCh. 1.2 - Prob. 1.2PCh. 1.2 - Judging from their relative positions in the...Ch. 1.2 - Classify each bond as nonpolar covalent or polar...Ch. 1.2 - Using the symbols and +, indicate the direction...Ch. 1.2 - Draw Lewis structures showing all valence...Ch. 1.2 - Draw Lewis structures for these ions and show...Ch. 1.3 - Draw Lewis structures and condensed structural...Ch. 1.3 - Prob. 1.9PCh. 1.3 - Prob. 1.10P
Ch. 1.3 - Prob. 1.11PCh. 1.3 - Prob. 1.12PCh. 1.4 - Predict all bond angles for these molecules. (a)...Ch. 1.5 - The geometry of carbon in diamond is tetrahedral,...Ch. 1.5 - Because of their spherical shape, C60 molecules...Ch. 1.5 - What best describes the CCC bond angles in C60? 1....Ch. 1.5 - Prob. 1.14PCh. 1.7 - Describe the bonding in these molecules in terms...Ch. 1.8 - Prob. 1.16PCh. 1.8 - Prob. 1.17PCh. 1.8 - Prob. 1.18PCh. 1.9 - Draw three contributing structures of the...Ch. 1.9 - What is the hybridization state of the circled...Ch. 1.9 - The molecule shown on the right in the example in...Ch. 1.9 - Prob. CQCh. 1.9 - The following structure is called imidazolium....Ch. 1 - Write the ground-state electron configuration for...Ch. 1 - Identify the atom that has each ground-state...Ch. 1 - Define valence shell and valence electron.Ch. 1 - How many electrons are in the valence shell of...Ch. 1 - Prob. 1.24PCh. 1 - Prob. 1.25PCh. 1 - Prob. 1.26PCh. 1 - Write Lewis structures for these compounds. Show...Ch. 1 - Write Lewis structures for these ions. Show all...Ch. 1 - Prob. 1.29PCh. 1 - Some of these structural formulas are incorrect...Ch. 1 - Following the rule that each atom of carbon,...Ch. 1 - Following are several Lewis structures showing all...Ch. 1 - Which statements are true about electronegativity?...Ch. 1 - Why does fluorine, the element in the upper right...Ch. 1 - Arrange the single covalent bonds within each set...Ch. 1 - Using the values of electronegativity given in...Ch. 1 - Prob. 1.37PCh. 1 - Use VSEPR to predict bond angles about each...Ch. 1 - Use VSEPR to predict bond angles about each atom...Ch. 1 - Use VSEPR to predict the geometry of these ions....Ch. 1 - Prob. 1.41PCh. 1 - Prob. 1.42PCh. 1 - What is the meaning of the term tertiary (3) when...Ch. 1 - What is the meaning of the term tertiary (3) when...Ch. 1 - Draw structural formulas for (a) The four primary...Ch. 1 - Draw structural formulas for the three tertiary...Ch. 1 - Prob. 1.47PCh. 1 - Identify the functional groups in each compound.Ch. 1 - Draw a three-dimensional representation for each...Ch. 1 - Tetrafluoroethylene, C2F4, is the starting...Ch. 1 - Which statements are true about resonance...Ch. 1 - Prob. 1.52PCh. 1 - Prob. 1.53PCh. 1 - Prob. 1.54PCh. 1 - Are the structures in each set valid contributing...Ch. 1 - State the orbital hybridization of each...Ch. 1 - Describe each highlighted bond in terms of the...Ch. 1 - Following is a structural formula of the...Ch. 1 - Draw a Lewis structure for methyl isocyanate,...Ch. 1 - What is the hybridization of the highlighted atoms...Ch. 1 - Using cartoon representations, draw a molecular...Ch. 1 - In what kind of orbitals do the lone-pair...Ch. 1 - Draw the delocalized molecular orbitals for the...Ch. 1 - Prob. 1.64APCh. 1 - Each compound contains both ions and covalent...Ch. 1 - Predict whether the carbon-metal bond in these...Ch. 1 - Prob. 1.67APCh. 1 - Phosphorus is immediately under nitrogen in the...Ch. 1 - Draw a Lewis structure for the azide ion, N3. (The...Ch. 1 - Cyanic acid, HOCN, and isocyanic acid, HNCO,...Ch. 1 - In Chapter 6, we study a group of organic cations...Ch. 1 - Many reactions involve a change in hybridization...Ch. 1 - Following is a structural formula of benzene,...Ch. 1 - Following are three contributing structures for...Ch. 1 - (a) Draw a Lewis structure for the ozone molecule,...Ch. 1 - The following two compounds are isomers; that is,...Ch. 1 - In future chapters, we will encounter...Ch. 1 - Prob. 1.78AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- There are two compounds with the molecular formula HN3. One is called hydrogen azide; the other is cyclotriazene. (a) Write the Lewis structure for each compound. (b) Designate the hybridization of each nitrogen in hydrogen azide. (c) What is the hybridization of each nitrogen in cyclotriazene? (d) How many sigma bonds are in hydrogen azide? In cyclotriazene? (e) How many pi bonds are in hydrogen azide? In cyclotriazene? (f) Give approximate values for the N-to-N-to-N bond angles in each molecule.arrow_forwardA useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, H3CCN. It is present in paint strippers. (a) Write the Lewis structure for acetonitrile, and indicate the direction of the dipole moment in the molecule. (b) Identify the hybrid orbitals used by the carbon atoms in the molecule to form bonds. (c) Describe the atomic orbitals that form the n bonds in the molecule. Note that it is not necessary to hybridize the nitrogen atom.arrow_forwardBoron trifluoride, BF3, reacts with ammonia, NH3, to form an addition compound, BF3NH3. Describe the geometries about the B and the N atoms in this compound. Describe the hybridization on these two atoms. Now describe the bonding between the B and N atoms, using valence bond theory. Compare the geometries and hybridization of these atoms in this addition compound with that in the reactant molecules BF3 and NH4.arrow_forward
- Consider the bonding in nitrate ion, NO3. First draw resonance formulas of this ion. Now describe the bonding of this ion in terms of molecular orbitals. (Refer to the delocalized bonding of the ozone molecule described in the text.) Suppose each atom uses sp2 hybrid orbitals. How many molecular orbitals can you form from the 2p orbitals that remain on these atoms? How many of these orbitals will be occupied?arrow_forwardFor each of the following structures, determine the hybridization requested and whether the electrons will be delocalized: (a) Hybridization of each carbon (b) Hybridization of sulfur (c) All atomsarrow_forwardIdentify the hybridization of each carbon atom in the following molecule. (The arrangement of atoms is given; you need to determine how many bonds connect each pair of atoms.)arrow_forward
- Cinnamaldehyde ocaus naturally in cinnamon oil. (a) What is the most polar bond in the molecule? (b) How many bonds and how many bonds are there? (c) Is cis-trans isomerism possible? If so, draw the isomers of the molecule. (d) Give the hybridization of the C atoms in the molecule. (e) What are the values of the bond angles 1, 2, and 3 ?arrow_forwardHow are the following similar, and how do they differ? (a) molecular orbitals and molecular orbitals (b) for an atomic orbital and for a molecular orbital (c) bonding orbitals and antibonding orbitalsarrow_forwardIn propene CH3CH=CH2, the first carbon has sp3 hybrid orbitals and the second carbon has sp2 hybrid orbitals. These orbitals interact to make a bond. Why are these hybrid orbitals not orthogonal?arrow_forward
- Identify any carbon atoms that change hybridization and the change in hybridization during the reactions in Exercise 20.39.arrow_forwardPelargondin is the molecule responsible for the red color of the geranium flower. It also contributes to the color of ripe strawberries and raspberries. The structure of pelargondin is: How many and bonds exist in pelargondin? What is the hybridization of the carbon atoms marked 14?arrow_forwardStrike-anywhere matches contain a layer of KClO3 and a layer of P4S3. The heat produced by the friction of striking the match causes these two compounds to react vigorously, which sets fire to the wooden stem of the match. KCIO3 contains the ClO3 ion. P4S3 is an unusual molecule with the skeletal structure. (a) Write Lewis structures for P4S3 and the ClO3 ion. (b) Describe the geometry about the P atoms, the S atom, and the Cl atom in these species. (c) Assign a hybridization to the P atoms, the S atom, and the Cl atom in these species. (d) Determine the oxidation states and formal charge of the atoms in P4S3 and the ClO3 ion.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY