
Concept explainers
(a)
Interpretation: The structure of the given compound para-toluidine has to be drawn.
Concept introduction:
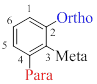
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)
Aryl
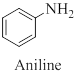
Toluidine is an aryl amine whose structure is similar to aniline except that a methyl group is substituted onto the benzene ring.
(b)
Interpretation: The structure of the given compound meta-cresol has to be drawn.
Concept Introduction:
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

Phenol is an
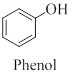
Cresol is a derivative of phenol which contains a methyl group other than the
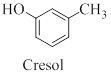
(c)
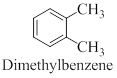
Interpretation: The structure of the given compound para-xylene has to be drawn.
Concept Introduction:
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

Dimethyl benzene compounds are known as xylene. Depends on the position of methyl group it can be exist as ortho, para and Meta compound.
(d)
Interpretation:
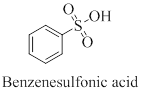
The structure of the given compound ortho-chlorobenzenesulfonic acid has to be drawn.
Concept introduction:
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

Benzene sulfonic acid is an organosulfur compound with the following structure.
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Organic Chemistry - MasteringChemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





