
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2, Problem 11P
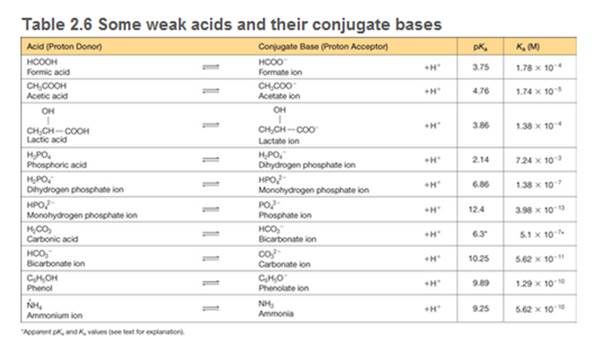
You need to make a buffer whose pH is 7.0, and you can choose from the weak acids shown in Table 2.6. Briefly explain your choice.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
To minimize the sharp pH shift that occurs when a strong acid is added to a solution, is it better to add a weak base or a strong base? Why?
If 4 mL of 1 M NaOH is added to 100 mL buffer, would it still be a usable buffer according to the conventions? Explain why or why not.
It is possible to make a buffer that functions well near pH7 using citric acid, which contains only carboxylate groups. Explain.
Chapter 2 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - A biochemical reaction takes place in a 1.00 ml...Ch. 2 - Is RNA-binding enzyme RNase A more likely to have...Ch. 2 - Consider a protein in which a negatively charged...Ch. 2 - Prob. 23PCh. 2 - Prob. 24P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- How much of a 10x buffer should be added to 900ml of water to make a 1x solution?arrow_forwardWhat is the pH of a buffer of a buffer system that contains a weak acid of .19 M and conjugate base of .12 M ? The Ka of the acid is 5.6x 10 -6 pH= pKa + log ( [A- ]/[HA]) please show me step by steparrow_forwardBased on the information in Table 3.2, is there any amino acid that could serve as a buffer at pH 8? If so, which one?arrow_forward
- A solution with a low pH means what?arrow_forwardWe usually say that a perfect buffer has its pH equal to its pKa. Give an example of a situation in which it would be advantageous to have a buffer with a pH 0.5 unit higher than its pKa.arrow_forwardHow much of a 5x buffer and how much water should be added to make 1 L of a 1x solution?arrow_forward
- The pH of a solution containing 20 mL of 0.120 N NaOH and 35 mL of 0.120 N NaOH is 1.49. True or False?arrow_forwardWhat is the pH of the following buffer mixtures? (a) 100 mL 1 M acetic acid plus 100 mL 0.5 M sodium acetate (b) 250 mL 0.3 M phosphoric acid plus 250 mL 0.8 M KH2PO4arrow_forwardAn aqueous solution contains 0.448 M dimethylamine ((CH3)2NH).How many mL of 0.240 M hydroiodic acid would have to be added to 150 mL of this solution in order to prepare a buffer with a pH of 10.500? ______________mlarrow_forward
- The pH scale is valid only for water. Why is this so?arrow_forwardThe pH of a 0.0082 M solution of HNO₃ isarrow_forwardYou are asked to design three buffers of the following pH values: 9.10, 10.45, and 4.65. You have the following 0.10M solutions available: CH3COOH, CH3COONa, NH4Cl, NH3, NaHCO3, and Na2CO3.Which solution will you choose to design each buffer?pH 4.65 ___________________________________________________ pH 9.10 ___________________________________________________ pH 10.45 __________________________________________________arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning


Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning

GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license