
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 20P
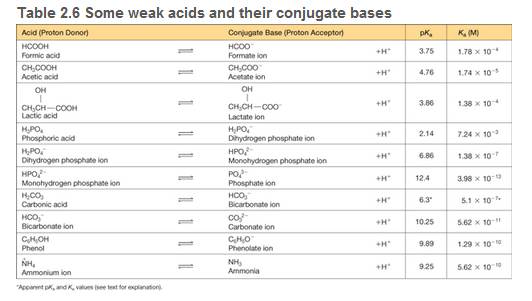
A biochemical reaction takes place in a 1.00 ml solution of 0.0250 M phosphate buffer initially at pH = 7.20 (see Table 2.6 for pkas of phosphate species).
a. Are the concentrations of any of the four possible phosphate species negligible? If so, identify them and explain your answer.
b. During the reaction, 3.80 µmol of HCI are produced. Calculate the final pH of the reaction solution. Assume that the HCI is completely neutralized by the buffer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
You have an initial solution in which you added quantities of “A” and “B” such that there is 4.5 M “A” and 2.5 M “B” and no complex (“AB”) at time 0. After equilibrium, you are able to isolate and quantitate the “AB” complex, and find its concentration is 1.5 M. Given that RT is 0.59 kcal/mol, what is the delta Go’ for the association reaction?
Nonspecific elution of affinity bonded macromolecules is used in affinity chromatography explain why?
The biochemical standard free energy change for the reaction:
A → B is −15.0 kJ/mol.
What is the equilibrium constant at 25°C and pH 7?
What is the free energy change for the reaction A→B at 37°C when [A]=10.0 mM and [B]=0.100 mM? (Give your answer in kJ/mol)
Chapter 2 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - A biochemical reaction takes place in a 1.00 ml...Ch. 2 - Is RNA-binding enzyme RNase A more likely to have...Ch. 2 - Consider a protein in which a negatively charged...Ch. 2 - Prob. 23PCh. 2 - Prob. 24P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Assume that the experiments performed in the absence of inhibitors were conducted by adding 5 μL of a 2 mg/mL enzyme stock solution to an assay mixture with a total volume of 1 mL. Take into account that XYZase is a monomeric enzyme with a molecular mass of 45,000 Daltons. Hint: To calculate the ???? in units of per second (s−1), you must first determine the ???? in micromoles per second (μmol/sec). Please explain step by steparrow_forwardThe ionization of p-nitrophenol is shown below (pKa = 7.0): a. Identify the weak acid and conjugate base. b. At pH 7, what are the relative concentrations of ionized and un-ionized p-nitrophenol? c. If enough concentrated hydrochloric acid is added to a solution of p-nitrophenol to lower the pH from 7 to 5, what will happen to the relative concentrations of the ionized and un-ionized forms? d. Ionized p-nitrophenol has a yellow color, while the un-ionized form is colorless. The yellow color can be measured using a spectrophotometer at 400nm. In order to determine the total amount of p-nitrophenol in a solution, would you perform the spectrophotometer reading at an acidic or basic pH? Clearly explain why? e. A solution of p-nitrophenol at pH 7.95 was found to have an A400 of 0.255 . What is the total concentration (in µM) of p-nitrophenol (ionized plus un-ionized) in the solution? The molar extinction coefficient of p-nitrophenol is 18,500 M-1cm-1 and the pKa is 7.arrow_forward1)Ubiquitin is a small protein with a monoisotopic mass of 8560 Da. a) Electrospray ionization of this small protein typically results in major charge states of +8, +9, +10, +11, +12, and +13. Using this information, complete the table below, assuming the charges on each come from protonation. Report mass and m/z values to the ones place. b) Using the data you entered in the table, sketch an expected ESI-MS spectrum for ubiquitin. Label each peak with its charge state. What do you notice about the spacing of peaks along the x-axis. c)The figure shows an experimentally obtained electrospray mass spectrum for ubiquitin. Compare this spectrum to the spectrum you predicted. Are there any differences? If so, what might cause these differences?arrow_forward
- Given the titration curve of the hypothetical polyprotic acid X at 0.100 M concentration (pKa1=4.0, pKa2=8.0, pKa3=12.0) titrated with 0.600 M NaOH, identify the pH at point C, H, E, and M.arrow_forwardThe amino acid glycine is often used as the main ingredient of a bufferin biochemical experiments. The amino group of glycine, which has apKa of 9.6, can exist either in the protonated form. (a) In what pH range can glycine be used as an effective buffer due to itsamino group?(b) When 99% of the glycine is in its protonated form, what is the numerical relation between the pH of the solution and the pKa of the amino group?arrow_forwardFrom a kinetics experiment, the Vmax was determined to be 450µM∙min-1. For the kinetic assay, 0.1mL of a 0.05mg/mL solution of enzyme was used, and the enzyme has a molecular weight of 125,000 g/mole. Assume a reaction volume of 700µL. Calculate the kcat (in sec-1) for the enzyme.arrow_forward
- Enzyme X has a molecular weight of 48,000. It converts substrate Z into product Y. Z absorbs at 340 nm, and Y absorbs at 480 nm. A.) At what wavelength would you measure the change in absorbance to assay for enzyme X? Would the absorbance increase or decrease over time? B.)If Vmax = 60 μmol/min and you used 400 μL of a 0.1 mg/mL solution of enzyme, what is the turnover number?arrow_forwardUsing a detailed scheme, propose a step-wise protocol to purify protein B by ion exchange chromatography (Explain your logic/choices).arrow_forwardConsider the nonenzymatic elementary reaction A → B. When the concentration of A is 20 mM, the reaction velocity is measured as 5 μM B produced per minute. (a) Calculate the rate constant for this reaction. (b) What is the molecularity of the reaction?arrow_forward
- Chitinase is a protein that breaks down chitin, a primary component of the cell wall in fungi, scales in fish and exoskeletons of arthropods. The activity of chitinase extracted from a plant was shown to be optimum at pH 5. You were tasked to prepare 300 mL of 150 mM buffer solution for further analysis of the extracted chitinase. REAGENTS Ka 2.5M Acetic acid Solid NaOAc•3H2O [136.08g/mol] 1.76 x 10-5 2.5M NH3 Solid NH4Cl [53.49g/mol] 5.6 x 10-10 2.5M Lactic acid Solid sodium lactate [112.06g/mol] 4.0 x 10-5 5 M HCl 5M NaOH Pls show sol'ns 1. Given the following reagents, give the moles of each component (acid & base).2. What are the mass/volume of the components needed to prepare the buffer? 3. What will the pH of the buffer be if 1mL of 5 M NaOH was added?arrow_forwardIn many biochemical reactions which involves the formation of an enolate intermediate, the carbonyl oxygen of the substrate is coordinated to a divalent metal ion (usually zinc or magnesium) in the active site. Explain with structural drawings, how this ion-dipole interactions affect the acidity of the a-protons?arrow_forwardConsider a buffer solution that contains 0.55 M NH2CH2CO2H and 0.35 M NH2CH2CO2Na. pKa(NH2CH2CO2H)=9.88. a. Calculate its pH. b. Calculate the change in pH if 0.155 g of solid NaOH is added to 250 mL of this solution. c. If the acceptable buffer range of the solution is ±0.10 pH units, calculate how many moles of H3O+ can be neutralized by 250 mL of the initial buffer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Enzyme Kinetics; Author: MIT OpenCourseWare;https://www.youtube.com/watch?v=FXWZr3mscUo;License: Standard Youtube License