
Concept explainers
(a)
Interpretation: The product of the given proton transfers reaction is to be drawn. The acid and base in the starting material, and the conjugate acid and base in the products are to be stated.
Concept introduction: In proton transfer reaction, acid donate protons to from a conjugate base and a base accepts protons to from a conjugate acid. An acid base reaction is represented as,
Answer to Problem 2.40P
The products of the given proton transfer reaction are
Explanation of Solution
The given starting material
The products of the given proton transfer reaction are
(b)
Interpretation: The product of the given proton transfers reaction is to be drawn. The acid and base in the starting material, and the conjugate acid and base in the products are to be stated.
Concept introduction: In proton transfer reaction, acid donate protons to from a conjugate base and a base accepts protons to from a conjugate acid. An acid base reaction is represented as,
Answer to Problem 2.40P
The products of the given proton transfer reaction are
Explanation of Solution
The given starting material
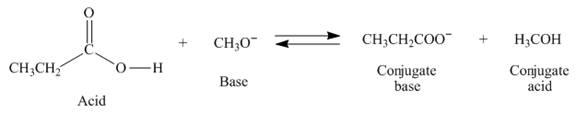
Figure 1
The products of the given proton transfer reaction are
(c)
Interpretation: The product of the given proton transfers reaction is to be drawn. The acid and base in the starting material, and the conjugate acid and base in the products are to be stated.
Concept introduction: In proton transfer reaction, acid donate protons to from a conjugate base and a base accepts protons to from a conjugate acid. An acid base reaction is represented as,
Answer to Problem 2.40P
The products of the given proton transfer reaction are
Explanation of Solution
The given starting material
The products of the given proton transfer reaction are
(d)
Interpretation: The product of the given proton transfers reaction is to be drawn. The acid and base in the starting material, and the conjugate acid and base in the products are to be stated.
Concept introduction: In proton transfer reaction, acid donate protons to from a conjugate base and a base accepts protons to from a conjugate acid. A acid base reaction is represented as,
Answer to Problem 2.40P
The products of the given proton transfer reaction are
Explanation of Solution
The given starting material
The products of the given proton transfer reaction are
Want to see more full solutions like this?
Chapter 2 Solutions
ALEKS 360 CHEMISTRY ACCESS
- For the previous four questions, label each molecule that appears in the question or your answer asstrong acid, strong base, weak acid, or weak base.arrow_forwardDraw the most stable conjugate base for each compund below (one hydrogen removed)arrow_forwardOChem question regarding acidity Consider ONLY the hydrogen drawn in each compound, list the compounds in order of INCREASING acidity. Also, explain why the most acidic is considered the most acidic and why the least acidic is considered least acidic. Thank you!arrow_forward
- Indicate the strongest acid for each pair of molecules: choose A or barrow_forwardA) for each compound show its conjugate base. lone pairs have been left out. B) rank the conjugate base in the order you would predict, from most to least stable. C) rank the original compounds in order, from strongest to weakest acid.arrow_forwardAcid plus base makes?arrow_forward
- a) Circle, or where appropriate, draw in the hydrogen(s) on the compounds that are the most acidic. b) Briefly explain your answers for each.arrow_forwardWhich one is the strongest base?arrow_forwardPropoxide (CH3CH2CH2O- ) is a larger molecule than ethoxide (CH3CH2O- ), yet they are equally basic. Explain why they are equally basic.arrow_forward
- How to do A?arrow_forwardPropranolol is an antihypertensive agent – that is, it lowers blood pressure. (a) Which proton in propranolol is most acidic? (b) What products are formed when propranolol is treated with NaH? (c) Which atom is most basic? (d) What products are formed when propranolol is treated with HCl?arrow_forwardAnswer the following questions about esmolol, a drug used to treat high blood pressure sold under the trade name Brevibloc.a.Label the most acidic hydrogen atom in esmolol. b.What products are formed when esmolol is treated with NaH? c.What products are formed when esmolol is treated with HCl? d. Label all sp2 hybridized C atoms. e.Label the only trigonal pyramidal atom. f.Label all C's that bear a δ+ charge.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

