
Concept explainers
(a)
Interpretation:
Lewis structure, VSEPR formula, bond angle, and molecular shape for
Concept Introduction:
Valence Shell Electron Pair Repulsion model predicts shape by inclusion of bond angles and most distant arrangement of atoms that leads to minimum repulsion. For the molecules that have no lone pairs around the central atom the bonded-atom unshared -pair arrangement is decided by the table as follows:
In order to determine the shape the steps to be followed are indicated as follows:
- 1. Lewis structure of molecule should be written.
- 2. The type electron arrangement around the central atom should be identified around the central atom. This essentially refers to determination of bond pairs and unshared or lone pairs around central atoms.
- 3. Then bonded-atom unshared -pair arrangement that can maximize the distance of electron pairs about central atom determines the shape.
For molecules that have lone pairs around central atom, lone pairs influence shape, because there are no atoms at the positions occupied by these lone pairs. The key rule that governs the molecular shape, in this case, is the extent of lone –lone pair repulsions are far greater than lone bond pair or bond pair-bond pair repulsions. The table that summarized the molecular shapes possible for various combinations of bonded and lone pairs are given as follows:
(a)
Answer to Problem 2E.16E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each atom in
The skeleton structure in
These 15 electron pairs are allotted as lone pairs to satisfy respective octets. Hence, the Lewis structure in
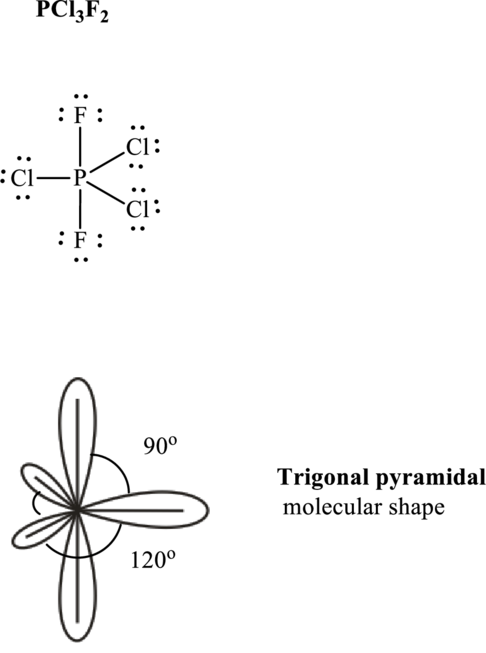
It is evident that
One lone pair is localized on equatorial positions so as to minimize lone pair–bond pair repulsions in accordance with VSPER model. This leads see-saw shape for
If lone pairs are represented by E, central atom with A and each unique atom attached by X and
(b)
Interpretation:
Lewis structure, VSEPR formula, bond angle, and molecular shape for
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 2E.16E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on atom in
The skeleton structure in
These 12 electron pairs are allotted as lone pairs to satisfy respective octets. Hence, the Lewis structure in
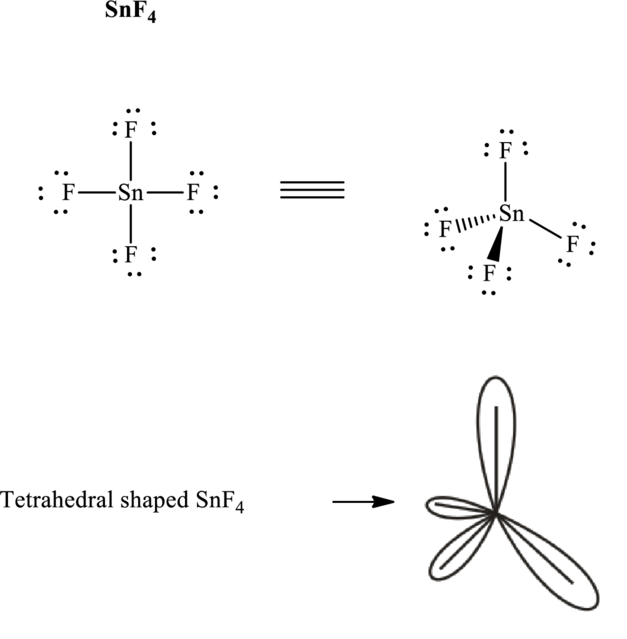
It is evident that
If lone pairs are represented by E, central atom with A and other attached bon pairs by X, then for any tetrahedral species with no one pairs the VSEPR formula is predicted to be
(c)
Interpretation:
Lewis structure, VSEPR formula, bond angle, and molecular shape for
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 2E.16E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on atom along with two negative charges in
The skeleton structure in
These 18 electron pairs are allotted as lone pairs on each fluorine atom to satisfy respective octets. Hence, the Lewis structure in
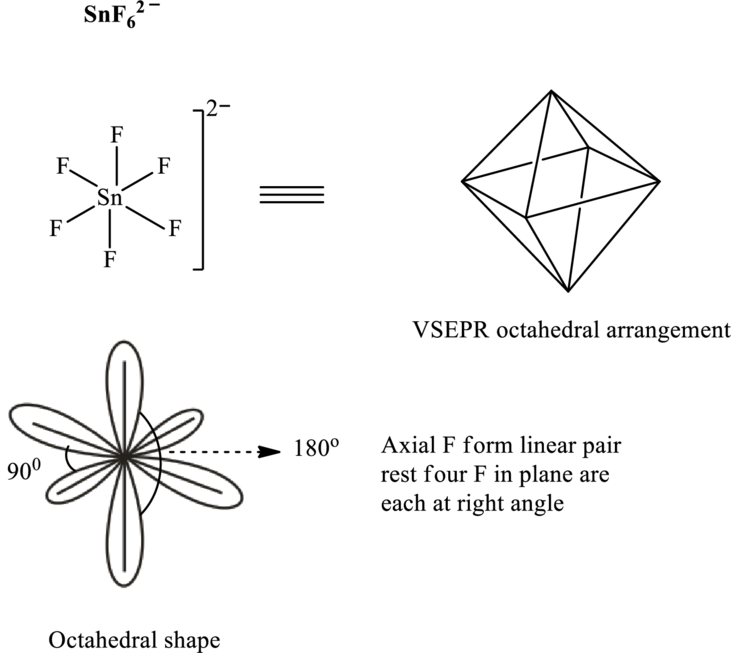
It is evident that in
(d)
Interpretation:
Lewis structure, VSEPR formula, bond angle and molecular shape for
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 2E.16E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each fluorine and central iodine in
The skeleton structure in
These 16 electron pairs are allotted as lone pairs of each of the fluorine atoms and one on central iodine to satisfy respective octet. Hence, the Lewis structure
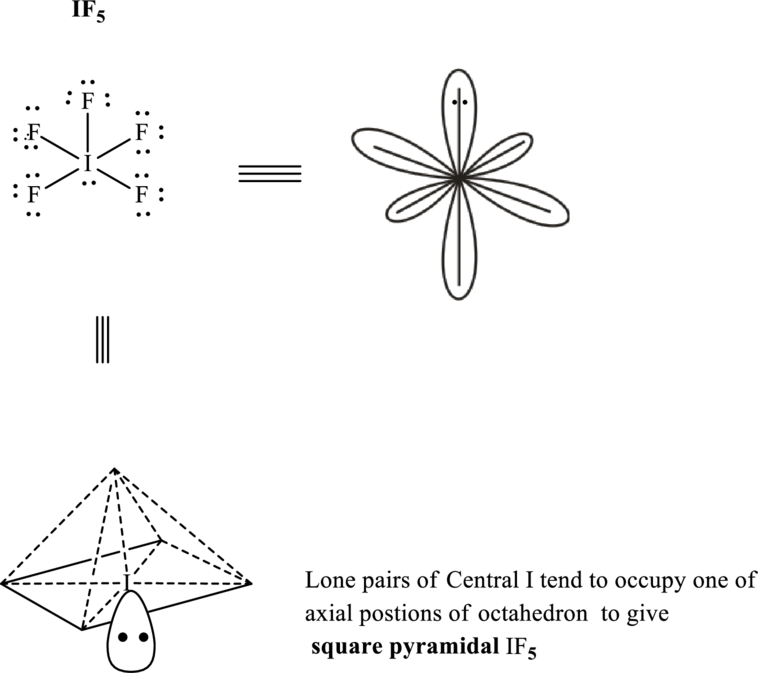
It is evident that in
If lone pairs are represented by E, central atom with A and other attached bond pairs by X, then for any square planar species the VSEPR formula is predicted as
(e)
Interpretation:
Lewis structure, VSEPR formula, bond angle and molecular shape for
Concept Introduction:
Refer to part (a).
(e)
Answer to Problem 2E.16E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on atom in
Thus, Lewis structure in
These 12 electron pairs are allotted as either lone pairs or multiple bonds with
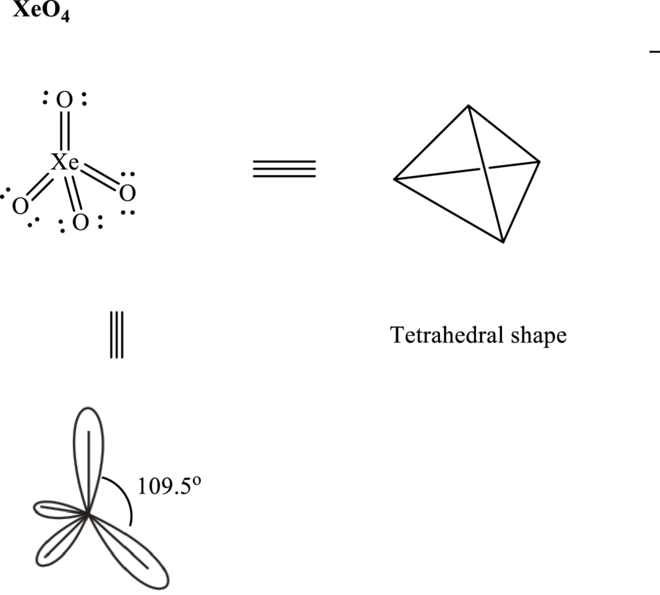
It is evident that in
So
If lone pairs are represented by E, central atom with A and other attached bond pairs by X, then for any tetrahedral species the VSEPR formula is predicted as
Want to see more full solutions like this?
Chapter 2 Solutions
CHEMICAL PRINCIPLES W/SAPLING
- Histidine is an essential amino acid that the body uses to form proteins. The Lewis structure of histidine follows. What are the approximate values for bond angles 1 through 5 (indicated on the structure by blue numbers)?arrow_forwardIt is possible to write a simple Lewis structure for the SO42- ion, involving only single bonds, which follows the octet rule. However, Linus Pauling and others have suggested an alternative structure, involving double bonds, in which the sulfur atom is surrounded by six electron pairs. (a) Draw the two Lewis structures. (b) What geometries are predicted for the two structures? (c) What is the hybridization of sulfur in each case? (d) What are the formal charges of the atoms in the two structures?arrow_forwardDraw the Lewis structure of ClF₃ (with minimized formal charges) and then determine its electron domain and molecular geometries.arrow_forward
- Except for nitrogen, the elements of Group 5A(15) all form pentafluorides, and most form pentachlorides. The chlorine atoms of PCl₅ can be replaced with fluorine atoms one at a timeto give, successively, PCl₄F, PCl₃F₂, ... , PF₅. (a) Given the sizesof F and Cl, would you expect the first two F substitutions to beat axial or equatorial positions? Explain. (b) Which of the five fluorine-containing molecules have no dipole moment?arrow_forwardDraw the Lewis structure of periodate (IO₄⁻) with minimized formal charges and then determine the hybridization of the central atom.arrow_forwardA molecule of formula AY3 is found experimentally to be polar. Which molecular shapes are possible and which are impossible for AY3?arrow_forward
- Draw the Lewis structure of HClO₃ (with minimized formal charges) and then choose the appropriate pair of molecular geometries of the two central atoms. Your answer choice is independent of the orientation of your drawn structure.arrow_forwardThe hybrid orbitals used in the central atom of AsF4+ is?arrow_forwardwhat are the bond angles of AsF4Br relative to AsF5? and why? how do the molecule structures differ?arrow_forward
- There are three possible structures for PCl2F3 with phosphorus as the central atom. Draw them and discuss how measurements of dipole moments could help distinguish among them.arrow_forwardUse electron-dot diagrams and formal charges to predict the bond orderfor each bond in SOF4 .arrow_forwardDraw the Lewis structure of SOF₄ (with minimized formal charges) and then determine the hybridization of the central atom.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

