
EP BIOLOGY TODAY+TOMORROW W/O PHYSIO.
5th Edition
ISBN: 9781305583856
Author: STARR
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 4FIO
Figure 2.17 Fatty acids.
Double bonds in the tails are highlighted in red.
- A. The tail of stearic acid is fully saturated with hydrogen atoms.
- B. Linoleic acid, with two double bonds, is unsaturated. The first double bond occurs at the sixth carbon from the end of the tail, so linoleic acid is called an omega-6 fatty acid. Omega-6 and
- C. omega-3 fatty acids are “essential fatty acids,” which means your body does not make them and they must come from food.
- D. The hydrogen atoms around the double bond in oleic acid are on the same side of the tail. Most other naturally occurring unsaturated fatty acids have these cis bonds.
- E. Hydrogenation creates abundant trans bonds, with hydrogen atoms on opposite sides of the tail.
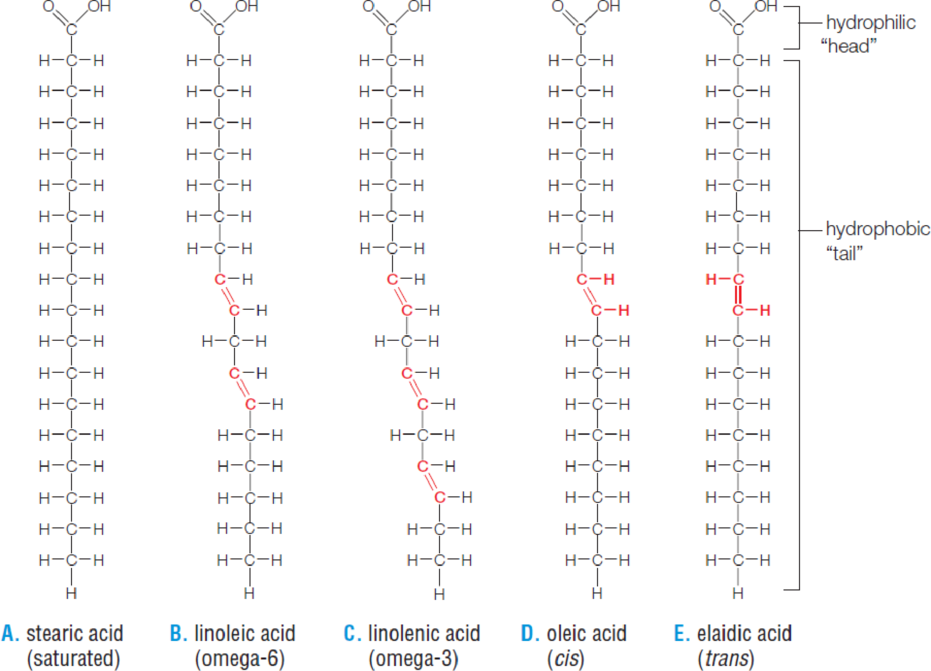
Figure It Out: Are the double bonds in linolenic acid cis or trans?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following best describes the fatty acid 20:3n-4?
A. There are 20 carbons plus three carboxyl group in the molecule.
B. It is a saturated fatty acid.
C. The molecule is an ω4 fatty acid.
D. 20:3Δ4,7,10,13.
E. It is a monounsaturated fatty acid
Which of the following properties of a free fatty acid molecule would make it capable of producing the most energy when used as a fuel source, compared to other free fatty acids?
a) A large number of unsaturated bonds
b) Shorter hydrocarbon chain with a large number of carbonyl groups
c) Longer hydrocarbon chain with a large number of saturated bonds
d) Shorter hydrocarbon chain with a large number of saturated bonds
A fatty acid with one or more double bonds between carbon atoms:
is unsaturated.
has the maximum number of hydrogen atoms possible bonded to the carbon chain.
is saturated.
is saturated and has the maximum number of hydrogen atoms possible bonded to the carbon chain.
is unsaturated and has the maximum number of hydrogen atoms possible bonded to the carbon chain.
Chapter 2 Solutions
EP BIOLOGY TODAY+TOMORROW W/O PHYSIO.
Ch. 2 - A. The first shell corresponds to the first energy...Ch. 2 - B. A chlorine atom (Cl) becomes a negatively...Ch. 2 - Figure 2.12 A pH scale. Here, red dots signify...Ch. 2 - Figure 2.17 Fatty acids. Double bonds in the tails...Ch. 2 - Effects of Dietary Fats on Lipoprotein Levels...Ch. 2 - Effects of Dietary Fats on Lipoprotein Levels...Ch. 2 - Effects of Dietary Fats on Lipoprotein Levels...Ch. 2 - Prob. 1SQCh. 2 - Which element has only one proton?Ch. 2 - The mutual attraction of opposite charges holds...
Ch. 2 - A salt does not release __________ in water. a....Ch. 2 - A(n) _______ substance repels water. a. acidic b....Ch. 2 - When dissolved in water, a(n) _____ donates H+ and...Ch. 2 - _________ is a monosaccharide. a. Glucose b....Ch. 2 - Unlike saturated fatty acids, the tails of...Ch. 2 - Which of the following is a class of molecules...Ch. 2 - Prob. 10SQCh. 2 - Prob. 11SQCh. 2 - Prob. 12SQCh. 2 - Prob. 13SQCh. 2 - Match the molecules with the best description.Ch. 2 - Match each molecule with its component(s).Ch. 2 - Alchemists were the forerunners of modern-day...Ch. 2 - Prob. 2CTCh. 2 - Polonium is a rare element with 33 radioisotopes....Ch. 2 - In the following list, identify the carbohydrate,...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Fill in the blanks. The parentheses are the choices for the blank. ______ (Saturated fatty acids, Unsaturated fatty acids). are fatty acids with only single bonds linking the carbons in its tail. A ______ (triglyceride, steroid, phospholipid) is a lipid with three fatty acid tails bonded to a glycerol.arrow_forwardFatty acids are esterified into mono-, di-, or triglycerides by attaching to: Sterol Cholesterol Glycerol Sodium chloride Omega-3 fatty acids have which of the following? At least 3 double bonds A 3-carbon backbone A double bond 3 carbons from the end of the chain At least 3 fatty acid molecules linked by omega bondsarrow_forwardWhich is NOT true about fatty acids? Select the correct answer below: In a triglyceride, the fatty acids are attached to each of the three carbons of the glycerol molecule with an ester bond through an oxygen atom Fatty acids have a long chain of hydrocarbons to which a ketone group is attached Fatty acids most commonly contain 12–18 carbons The number of carbons in a fatty acid may range from 4 to 36. Please explain eacharrow_forward
- Which of the following statements is true of fatty acids? Group of answer choices The more carbon atoms and nitrogen atoms present in a fatty acid, the shorter the fatty acid is. The more hydrogen atoms attached to carbon atoms, the more saturated a fatty acid is. All fatty acids are solid at room temperature. All fatty acids have only double bonds. All fatty acids have the same chain length.arrow_forwardIn which of the following pairs of fatty acids does the first listed acid have a higher solubility in water than the second listed acid? A. 18:0 acid and 18:3 acid B. 14:3 acid and 20:0 acid C. 12:0 acid and 22:0 acid D. 16:2 acid and 16:0 acidarrow_forwardWhich of the following is/are NOT (an) essential fatty acids? A. arachidonic acid B. linoleic acid C. palmitic D. oleic acidarrow_forward
- A fatty acid designated w-3A) has three double bonds.B) is saturated.C) has a double bond three carbons from the end of the chain.D) has a double bond three carbons from the a-carbon.arrow_forwardTRUE OR FALSE PROPERTIES OF FATTY ACIDS Contains polar hydrocarbon and non-polar carboxyl groups Carboxyl portion undergoes halogenation Forms ester bond on the hydrocarbon chain PROPERTIES OF POLYUNSATURATED FATS Forms single bonds and double bonds with hydrogen Undergo hydrogenation on bonds saturated with hydrogen Hardens when saturated with hydrogenarrow_forwardWhich is NOT a feature of a fatty acid? Even numbers of carbon atoms Can be saturated or unsaturated Straight chain with no branching Double bonds can be conjugatedarrow_forward
- For no. 1–2, refer to the figure below. 1. Which part of the fatty acid will likely interact with water? A. 1 B. 2 C. Both 1 and 2 D. Either 1 or 2 2. Which part of the fatty acid will likely interact with hexane? A. 1 B. 2 C. Both 1 and 2 D. Either 1 or 2arrow_forwardDuring hydrogenation, cis double bonds are converted to trans double bonds. In the lab, we compare three fats, each of which has fatty acid chains that are exactly the same length (number of carbons) and observe the following: Fat 1 contains only saturated fatty acids 16 carbons long, and has a melting point of 65 degrees C. Fat 2 contains only cis unsaturated fatty acids 16 carbons long, and has a melting point of 35 degrees C. Fat 3 contains only trans fatty acids 16 carbons long, and has a melting point of 65 degrees C. Both Fat 2 and Fat 3 contain fatty acids with a single double bond; fat 1 has no double bonds. Why do Fat 3 and Fat 1 have more similar melting points than Fat 3 and Fat 2? Group of answer choices The number of hydrogen atoms in the fatty acids of fats 1 & 3 is higher, and having more hydrogen atoms raises the melting point of the fat. The fatty acids in fats 1 & 3 have a linear shape, so they pack tightly together and have lots of hydrophobic…arrow_forwardIn a fatty acid, the shorter the carbon chain, the more solid the fatty acid becomes. True Falsearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning


Biology: The Unity and Diversity of Life (MindTap...
Biology
ISBN:9781305073951
Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Biology: The Unity and Diversity of Life (MindTap...
Biology
ISBN:9781337408332
Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license