
Concept explainers
Interpretation:
The procedure for separation of hexylamine from cyclohexane using dilute HCl, aqueous NaOH and diethyl ether is to be explained..
Concept Introduction:
On the basis of hydrogen atoms being replaced from ammonia by the organic groups, amines are of three types: primary amines
Answer to Problem 1PP
Solution: Hexylamine is soluble in dilute acids but cyclohexane is not.
Explanation of Solution
Given information: A solution of cyclohexane and hexylamine is to be separated by dilute acid, aqueous NaOH and diethyl ether.
Amines with larger number of carbon atoms are insoluble in water but they are soluble in dilute acids. Thus, by dissolving amines in acids, they can be separated easily. Amines carry a lone pair, which forms a bond with the hydrogen atoms of acids while, cyclohexane being non-polar does not interact with acids. The interaction of amines with dilute acid is shown below:
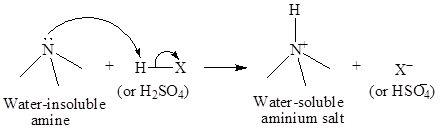
The separated amines can be extracted from acid by dissolving in aqueous acid solution and then recovered by making the aqueous solution basic and extracting the amine in etheror
Hexylamine can be separated from cyclohexane by dissolving the amine derivative in dilute acid solution.
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- Both pyridine and pyrrole are nitrogen containing aromatic heterocyclic compounds. When treated with HCl, only pyridine forms the hydrochloride salt, where as pyrrole is unreactive. What is the best explanation for this observed reactivity.arrow_forwardCompound A has the molecular formula C14H25Br and was obtained by reaction of sodium acetylide (HC≡CNa) )with 1,12-dibromododecane. On treatment of compound A with sodium amide, it was converted to compound B (C14H24). Ozonolysis of compound B gave the diacid HO2C(CH2)12CO2H. Catalytic hydrogenation of compound B over Lindlar palladium gave compound C (C14H26), while hydrogenation over platinum gave compound D (C14H28). Sodium-ammonia reduction of compound B gave compound E (C14H26). Both C and E yielded O═CH(CH2)12CH═O on ozonolysis. Assign structures to compound A through E so as to be consistent with the observed transformations.arrow_forwardProvide a stepwise synthesis for sodium benzoate to benzoic acid with reagents HCl (including arrow-pushing mechanisms) Kindly show all the arrow pusharrow_forward
- Provide a stepwise synthesis for benzyl chloride to benzoic acid (including arrow-pushing mechanisms) Benzyl Chloride - benzyl alcohol - sodium benzoate - benzoic acid Kindly show all the arrow pusharrow_forwardProvide a stepwise synthesis for benzyl chloride to benzoic acid. (including arrow-pushing mechanisms)arrow_forwardWhat is the name of the mechanism where camphor is reduced to isoborneol? Is it Nucleophillic/Electrophilic Addition/Substitution? Give explanation also.arrow_forward
- Triphenylmethanol is insoluble in water, but when it is treated with concentrated sulfuric acid, a bright yellow solutionresults. As this yellow solution is diluted with water, its color disappears and a precipitate of triphenylmethanol reappears.Suggest a structure for the bright yellow species, and explain this unusual behavior.1arrow_forwardProvide a stepwise synthesis for benzyl chloride to benzyl alcohol with reagents Na2CO3 and H2O (including arrow-pushing mechanisms) Kindly show all the arrow pusharrow_forward(−)-Hyoscyamine, an optically active drug used to treat gastrointestinal disorders, is isolated from Atropa belladonna, the deadly nightshade plant, by a basic aqueous extraction procedure. If too much base is used during isolation, optically inactive material is isolated. (a) Explain this result by drawing a stepwise mechanism. (b) Explain why littorine, an isomerisolated from the tailower plant in Australia, can be obtained optically pure regardless of the amount of base used during isolation.arrow_forward
- When the nitrogen-containing aromatic heterocyclic compounds 1 and 2 are treated with HCl, only 1 forms the hydrochloride salt, whereas compound 2 is unreactive. Provide an explanation for this observed reactivity.arrow_forward(a) Propose a reasonable synthesis for the formation of nonane from CH3CH2CH2I and any other organic/inorganic reagent. (b) Suggest three (3) different Grignard reactions leading to 2-phenyl-2-butanol.arrow_forward(a) Explain the mechanism of a nucleophilic attack on the carbonyl group of an aldehyde or a ketone.(b) An organic compound (A) (molecular formula CgH16Q2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid also produced (B). On dehydration (C) gives but-1-ene. Write the equations for the reactions involved.arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
