
Concept explainers
Interpretation: On addition of one mole of
Concept Introduction:
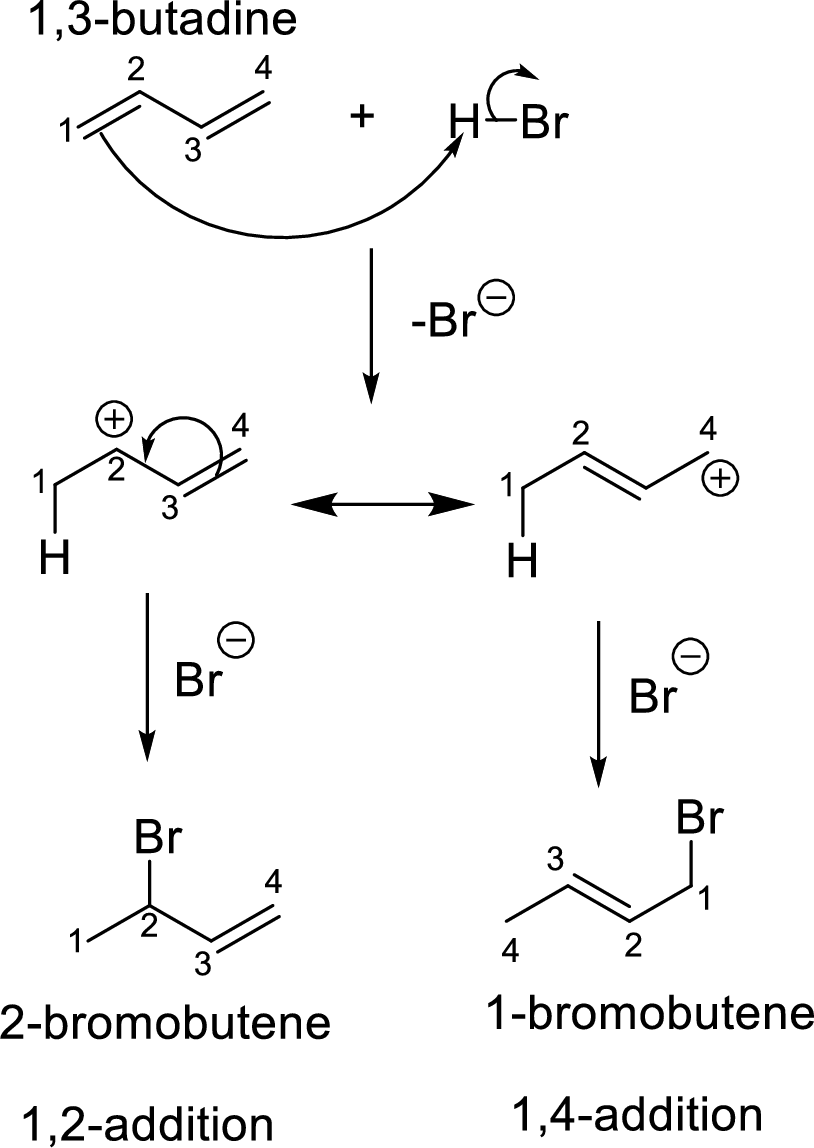
Addition reaction of dienes:
The diene with a four carbon system is
The constitutional isomers have same molecular formula and different connectivity of the substituents in the same structural formula.
Example:
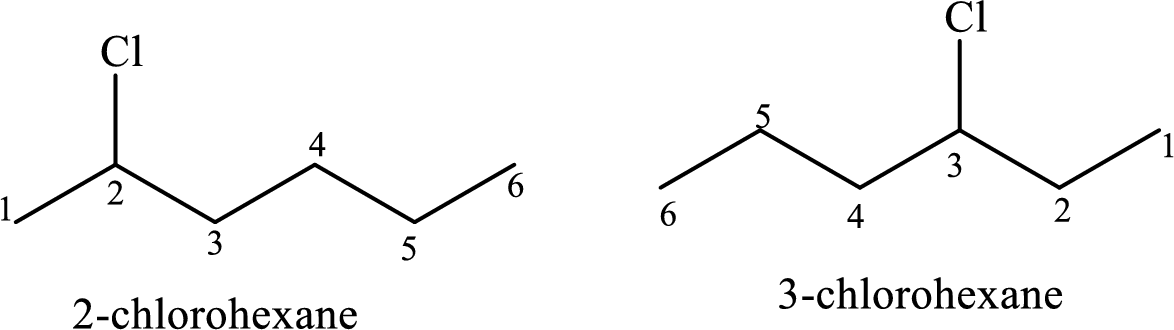
Trending nowThis is a popular solution!

Chapter 20 Solutions
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Classify alkyl halides as primary, secondary, tertiary, aliphatic, or benzenearrow_forwardExplain why the bond dissociation enthalpy of a C-H bond in benzene is significantly greater than that in alkanesarrow_forwardDraw the skeletal (bond-line) structures of all isomers of C4H8O (including configurational isomers) that contain an alkene and an ether. There should be 5 structures.arrow_forward
- Methyl acrylate (H2C=CHCO2CH3) reacts with 1,3-cyclopentadiene to give a mixture of two products. Write structural formulas for both and predict which one predominates.arrow_forwardWhen bromine is added to two beakers, one containing phenyl isopropyl ether and the other containing cyclohexene, the bromine color in both beakers disappears. What observation could you make while performing this test that would allow you to distinguish the alkene from the aryl ether?arrow_forwardWhen cyclopropane is treated with HI, 1-iodopropane is formed. A similar type of reaction does not occur with cyclopentane or cyclohexane. Suggest an explanation for cyclopropane’s reactivity.arrow_forward
- What is the molecular formula of 5-chloro-1-ethylcyclohexene? O C8H12CI none of these O C8H13CI O C₂H₁6CI O C6H₁4 CIarrow_forwardThe heat of combustion of cyclononane is 5586 kJ/mol (1335 kcal/mol). Calculate the ring strain per CH2 group and the total ring strain of cyclononane. Which compound has more ring strain, cyclononane or cycloheptane?arrow_forwardThe heat of combustion of decahydronaphthalene (C10H18) is -6286 kJ/mol. The heat of combustion of naphthalene 1C10H82 is -5157 kJ/mol. (In both cases CO2(g) and H2O(l) are the products). Calculate the heat of hydrogenation and the resonance energy of naphthalene.arrow_forward
- The heats of hydrogenation of cycloheptene and 1,3,5-cycloheptatriene are 110 kJ/mol (26.3 kcal/mol) and 305 kJ/mol (73.0 kcal/mol), respectively. In both cases cycloheptane is the product. What is the resonance energy of 1,3,5-cycloheptatriene? How does it compare with the resonance energy of benzene?arrow_forwardDraw the structural formula for a cycloalkene with the molecular formula C6H10 that reacts withCl2 in a halogenation reaction to give the following product.arrow_forwardGive at least 3 features in the structure of an alkene.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

