
Draw the product formed when
a.
![]() , then
, then
b.![]() , then
, then
c.
![]() , then
, then
d.
(a)
Interpretation: The product formed when
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.40P
The product formed when
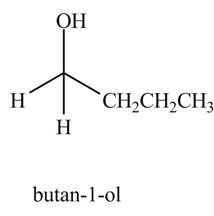
Explanation of Solution
The chemical equation when
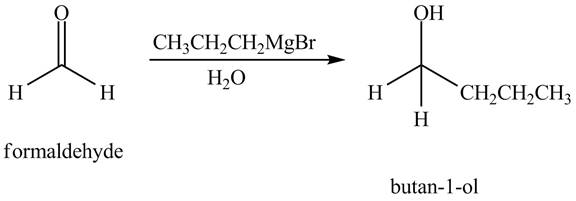
Figure 1
The above equation shows that formaldehyde reacts with
The product formed when
(b)
Interpretation: The product formed when
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.40P
The product formed when
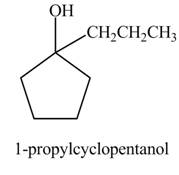
Explanation of Solution
The chemical equation when
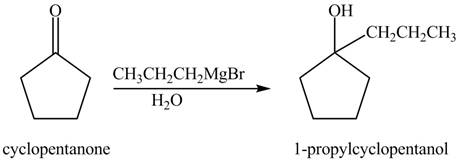
Figure 2
The above reaction indicates that that cyclopentanone reacts with
The product formed when
(c)
Interpretation: The product formed when
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.40P
The product formed when
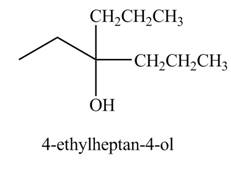
Explanation of Solution
The chemical equation when
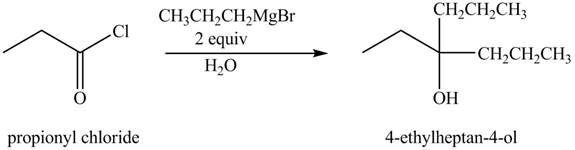
Figure 3
The above equation shows that propionyl chloride reacts with two equivalents of
The product formed when
(d)
Interpretation: The product formed when
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.40P
The product formed when
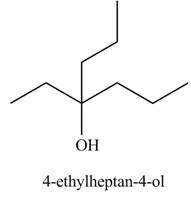
Explanation of Solution
The chemical equation when
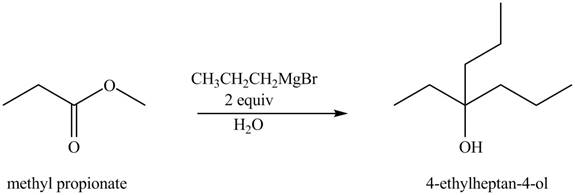
Figure 4
The above equation shows that methyl propionate reacts with two equivalents of
The product formed when
(e)
Interpretation: The product formed when
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.40P
The products formed when
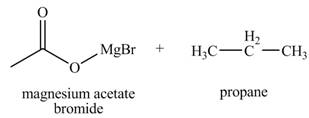
Explanation of Solution
The chemical equation when
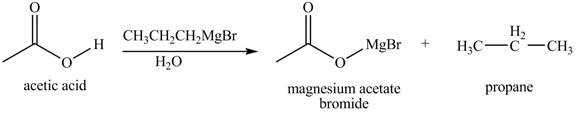
Figure 5
The above equation shows that acetic acid reacts with
The product formed when
(f)
Interpretation: The product formed when
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.40P
The products formed when
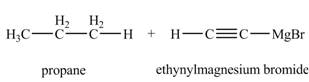
Explanation of Solution
The chemical equation when
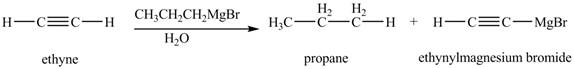
Figure 6
The above equation shows that ethyne reacts with
The product formed when
(g)
Interpretation: The product formed when
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.40P
The product formed when
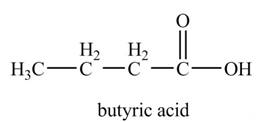
Explanation of Solution
The chemical equation when
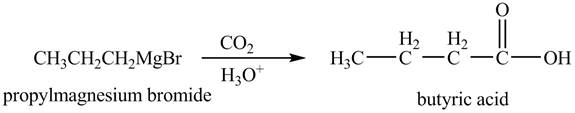
Figure 7
The above equation shows that propylmagnesium bromide reacts with
The product formed when
(h)
Interpretation: The product formed when
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.40P
The product formed when
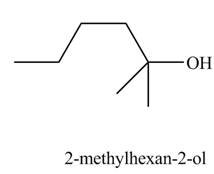
Explanation of Solution
The chemical equation when
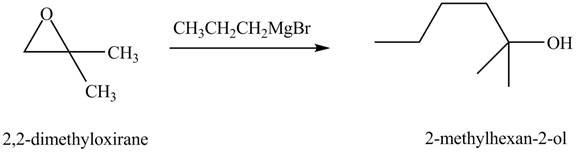
Figure 8
The above equation shows that
The product formed when
(i)
Interpretation: The product formed when
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.40P
The product formed when

Explanation of Solution
The chemical equation when
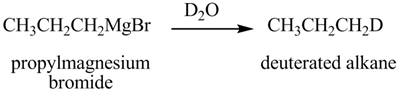
Figure 9
The above equation shows that propylmagnesium bromide reacts with
The product formed when
(j)
Interpretation: The product formed when
Answer to Problem 20.40P
The product formed when
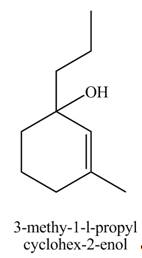
Explanation of Solution
The chemical equation when
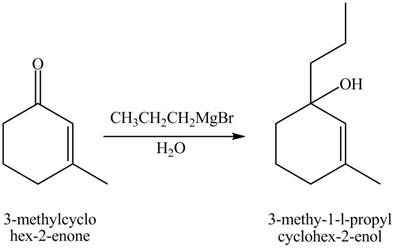
Figure 10
The compound
The product formed when
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry - With Access (Looseleaf) (Custom)
- From the given two compounds in the image, which reagents would differentiate the pair? a. Br2 in CH2Cl2 b. CrO3, H2SO4, acetone c. concentrated HCl with ZnCl2 d. aqueous FeCl3 e. aqueous NAHCO3 f. ammoniacal AgNo3arrow_forwardWhy is H3C-O-SiH3 ether a weaker base than (CH3)3O?arrow_forwardList the reagents necessary to produce the given product.arrow_forward
- Which of the following compounds is most soluble in water? a) CH3-CH2-CH2-CH2-COOH b) CH3-CH2-CH2-CH2-OH c)CH3-COOH d)CH3-CH2-CH3 e) CH3-CH2-CH2-O-CH3arrow_forwardWhat is the correct reagent?arrow_forwardLabel each statement as True or False. a. CH3CH2+ is the conjugate acid of CH2=CH2. b.CH3CH2− is the conjugate base of CH3CH2+. c. CH2=CH2 is the conjugate base of CH3CH2−. d. CH2=CH− is the conjugate base of CH2=CH2. e. CH3CH3 is the conjugate acid of CH3CH2−.arrow_forward
- which organic compound dissolves in water? 1. 2-pentanol 2. 1-hexanol 3. diethylether 4. 3-methyl-3-pentanol which organic compound dissolves in 10% NaOH? 1. 2-pentanol 2. 1-hexanol 3. diethylether 4. 3-methyl-3-pentanolarrow_forwardShow how to prepare each compound from 2-methyl- 1- propanol. a. 2- methylpropene b. 2- methyl- 2- propanol c. 2- methylpropanoic acid (CH3)2CHCOOHarrow_forwardAcetic Acid -> H+ + Acetate CH3COOH -> H+ + CH3COO- 1. What happens when sodium hydroxide is added to the solution? Do the relative concentrations of H+, CH3COOH, or CH3COO- change? 2. What happens when hydrochloric acid is added to the solution? Do the relative concentrations of H+, CH3COOH, or CH3COO- change? 3. Given that Ethanol does not interact with any of the chemicals listed in the initial reaction to change their relative concentration, what effect does it have on the solution?arrow_forward
- 1. What are the characteristics of a positive tollens test for adehydes? What is the oxidizing agent in tollens solutions? 2. What is the characteristics of a positive Benedict's test for aldehydes? What is the oxidizing agent in Benedict's solution?arrow_forwardwhich reagents complete the reaction?arrow_forwardDraw the products formed when D-altrose is treated with each reagent. a. (CH3)2CHOH, HCl b. NaBH4, CH3OH c. Br2, H2O d. HNO3, H2O e. [1] NH2OH; [2] (CH3CO)2O, NaOCOCH3; [3] NaOCH3 f. [1] NaCN, HCl; [2] H2, Pd-BaSO4; [3] H3O+ g. CH3I, Ag2O h. C6H5CH2NH2, mild H+arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


