
Concept explainers
(a)
Interpretation:
The product formed by the treatment of given compound with either
Concept introduction:
Organometallic reagents like
Answer to Problem 20.34P
The product formed by the treatment of given compound with
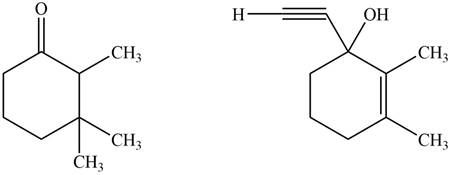
Figure 1
Explanation of Solution
The given reagents are organocuprate
The product formed by the reaction of
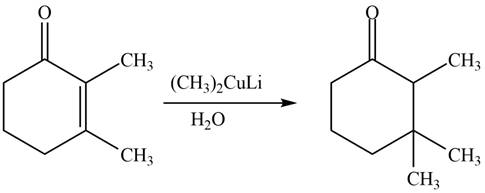
Figure 2
The product formed by the reaction of
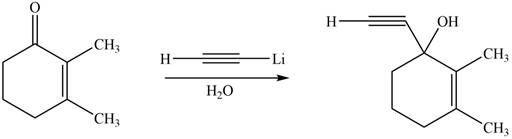
Figure 3
The product formed by the treatment of given compound with
(b)
Interpretation:
The product formed by the treatment of given compound with either
Concept introduction:
Organometallic reagents like
Answer to Problem 20.34P
The product formed by the treatment of given compound with
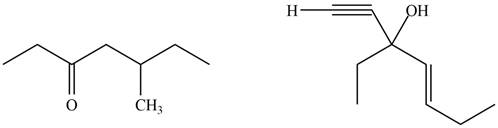
Figure 4
Explanation of Solution
The given reagents are organocuprate
The product formed by the reaction of
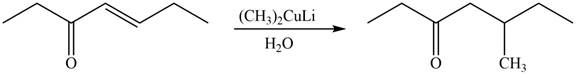
Figure 5
The product formed by the reaction of
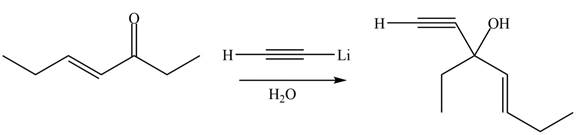
Figure 6
The product formed by the treatment of given compound with
(c)
Interpretation:
The product formed by the treatment of given compound with either
Concept introduction:
Organometallic reagents like
Answer to Problem 20.34P
The product formed by the treatment of given compound with
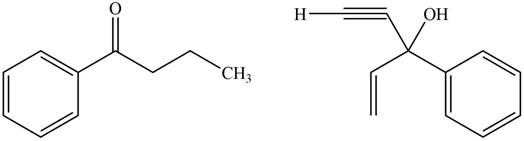
Figure 7
Explanation of Solution
The given reagents are organocuprate
The product formed by the reaction of
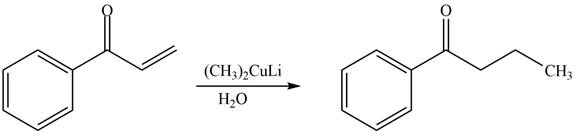
Figure 8
The product formed by the reaction of
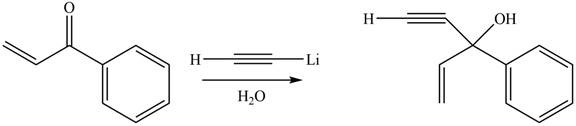
Figure 9
The product formed by the treatment of given compound with
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry - With Access (Looseleaf) (Custom)
- Draw the product formed when (CH3)2CHOH is treated with following reagent. PBr3, then NaCNarrow_forwardWhat product is formed when each compound is treated with either LiAlH4 (followed by H2O), or NaBH4 in CH3OH?arrow_forwardWhat products are formed when benzoic acid (C 6H 5COOH) is treated with each base: (a) NaOH; (b) Na 2CO 3; (c) NaHCO 3?arrow_forward
- Draw the product formed when A is treated with below series of reagents. [1] H2O; [2] NaH; [3] CH3Brarrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent. a.SOCl2, pyridine b. TsCl, pyridine c.H2SO4 d.HBr e.PBr3, then NaCN f.POCl3, pyridinearrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent. a. SOCl2, pyridine b. TsCl, pyridine c. H2SO4 d. HBr e. PBr3, then NaCN f. POCl3, pyridinearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





