
Concept explainers
What reagent is needed to convert
a.
b.
(a)
Interpretation:
The reagent needed to convert
Concept introduction:
Acid chlorides, which contains good leaving group
Answer to Problem 20.44P
The reagent needed to convert
Explanation of Solution
The given compound is aldehyde. Acid chlorides on reaction with mild reducing agent like lithium tri-tert-butoxyaluminium hydride
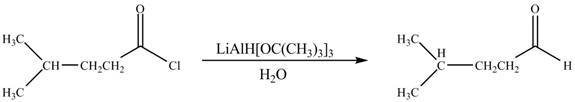
Figure 1
Therefore,
The reagent needed to convert
(b)
Interpretation:
The reagent needed to convert
Concept introduction:
Acid chlorides, which contains good leaving group
Answer to Problem 20.44P
The reagent needed to convert
Explanation of Solution
The structure of given ketone compound shows that one ethene molecule is attached to carbonyl carbon. Acid chlorides on reaction with one equivalent of organocuprate reagent convert into ketone. The organocuprate reagent removes the good leaving
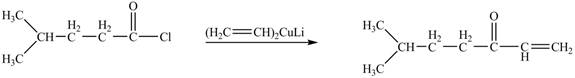
Figure 2
Therefore,
The reagent needed to convert
(c)
Interpretation:
The reagent needed to convert
Concept introduction:
Acid chlorides, which contains good leaving group
Answer to Problem 20.44P
The reagent needed to convert
Explanation of Solution
The structure of given ketone compound shows that the hydroxyl group is attached to tertiary carbon atom. Acid chlorides on reaction with two equivalents of Grignard reagent convert into tertiary alcohol. The first equivalent of Grignard reagent remove good leaving
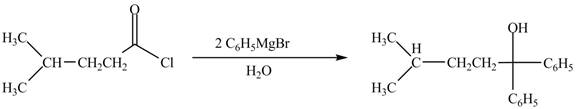
Figure 3
Therefore,
The reagent needed to convert
(d)
Interpretation:
The reagent needed to convert
Concept introduction:
Acid chlorides, which contains good leaving group
Answer to Problem 20.44P
The reagent needed to convert
Explanation of Solution
Acid chlorides on reaction with strong reducing agent like lithium aluminium hydride
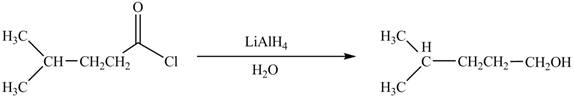
Figure 4
Therefore,
The reagent needed to convert
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry - With Access (Looseleaf) (Custom)
- What reagent is necessary to complete the reaction? CH3-CH2-CH-C-OH ? CHỊCH,CH C-0- Na* CH3 CH3 NaO O NaCl O Na O NaOH + H₂Oarrow_forwardRank the alcohols in order of increasing reactivity when dehydrated with H2SO4.arrow_forwardGive a common name (when possible) and a systematic name for each compound.(a) CH3OCH“CH2arrow_forward
- Rank the following groups in order of decreasing priority. a. – COOH, – H, – NH2, – OH b. – H, – CH3, – Cl, – CH2CI c. -CH2CH3, -CH3, -H, -CH(CH3)2 d. – CH = CH2, – CH3, – C ≡ CH, – Harrow_forward1. Which compound is more soulable in water : CH3 CH2 CH2 CH2 CH2 CH3 OR CH3 CH2 CH2 CH2 COOH 2. Which compound is more soulable in organic solvent : CH3 CH2 CH2 CH2 CH2 CH3 OR CH3 CH2 CH2 CH2 COOHarrow_forward(a) Draw the products formed when propane is heated with Br2. (b) Label major and minor products and explain with an energy diagram.arrow_forward
- What reagent can be used from compound F to G? (NaH / NaOH / LiAlH4+hydronium quench / CrO3 Jones)arrow_forwardWhich reagents are used for reaction 1? a NaN3, ethanol and NaBH4, ethanol, H3O+ b CH3CH2NH2, NaOH c NH3, NaOH d NH3, DCC Which reagent are used for reaction 2? a CH3COOH, DCC b CH3COCH3 and NaBH4, ethanol, H3O+ c CH3COH and NaBH4, ethanol, H3O+ d CH3CONH2 and CH3CH2NH2arrow_forwardWhat happens when(i) CH3—Cl is treated with aqueous KOH?(ii) CH3—Cl is treated with KCN?(iii) CH3—Br is treated with Mg in the presence of dry ether?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





