
An interesting pair of consecutive reactions involve the absorption of ethyl alcohol by the body, which is a first-order process, and the consequent oxidation of alcohol to acetaldehyde by liver alcohol dehydrogenase (LADH), which is a zeroth order process. The differential changes in the three states of ethanol can therefore be described as
which are slightly modified from equations
(a)
Interpretation:
The species
Concept introduction:
In the consecutive reactions the product of first reaction acts as the reactant for the second reaction and so on. The radioactive decays are one of the examples of the consecutive reactions. The simple two step consecutive reaction is shown below.
Answer to Problem 20.57E
The species
Explanation of Solution
The given consecutive reaction involves the absorption of ethyl alcohol by the body which is a first order reaction followed by the oxidation of ethyl alcohol to acetaldehyde in the presence of liver alcohol dehydrogenase (LADH).
The rate of change of concentration of the three species is given below.
From the above consecutive reaction it is concluded that
The species
(b)
Interpretation:
The integrated form for
Concept introduction:
In the consecutive reactions, the product of first reaction acts as the reactant for the second reaction and so on. The radioactive decays are one of the examples of the consecutive reactions. The simple two step consecutive reaction is shown below.
Answer to Problem 20.57E
The integrated form for
Explanation of Solution
The differential change in the concentration of
The integrated rate law for the first order reaction of
Substitute equation (2) in equation (1).
Integrate the above equation.
The integrated form for
(c)
Interpretation:
The integrated form for
Concept introduction:
In the consecutive reactions the product of first reaction acts as the reactant for the second reaction and so on. The radioactive decays are one of the examples of the consecutive reactions. The simple two step consecutive reaction is shown below.
Answer to Problem 20.57E
The integrated form for
Explanation of Solution
The differential change in the concentration of
The integrated form for
Substitute equation (4) in equation (3).
Integrate the above equation.
The above exponential function is integrated using the identity shown below.
On applying this identity on the exponential function the expression for the integrated form for
The integrated form for
(d)
Interpretation:
The graph for
Concept introduction:
In the consecutive reactions the product of first reaction acts as the reactant for the second reaction and so on. The radioactive decays are one of the examples of the consecutive reactions. The simple two step consecutive reaction is shown below.
Answer to Problem 20.57E
The graph for
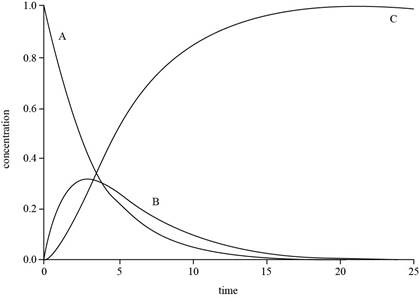
Explanation of Solution
The expressions for
The value of
The plot for
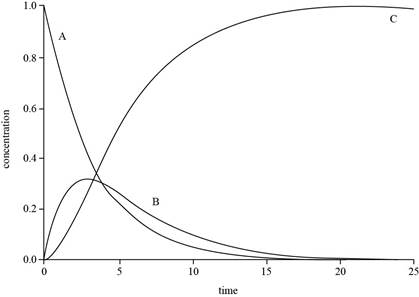
Figure 1
The concentration of
The graph for
Want to see more full solutions like this?
Chapter 20 Solutions
Physical Chemistry
- When enzymes are present at very low concentration, their effect on reaction rate can be described by first-order kinetics. Calculate by what factor the rate of an enzyme-catalyzed reaction changes when the enzyme concentration is changed from 1.5 107 M to 4.5 106 M.arrow_forwardExplain why half-lives are not normally used to describe reactions other than first order.arrow_forwardFor the past 10 years, the unsaturated hydrocarbon 1, 3-butadiene (CH2 = CH - CH = CH2) has ranked 38th among the top 50 industrial Chemicals. It is used primarily for the manufacture of synthetic rubber. An isomer exists also as cyclobutene: The isomerization of cyclobutene to butadiene is first-order and the rate constant has been measured as 2.0104s1 at 150 C in a 0.53-L ?ask. Determine the partial pressure of cyclobutene and its concentration after 30.0 minutes if an isomerization reaction is carried out at 150 C with an initial pressure of 55 torr.arrow_forward
- The hydrolysis of the sugar sucrose to the sugars glucose and fructose, C12H22O11+H2OC6H12O6+C6H12O6 follows a first-order rate equation for the disappearance of sucrose: Rate =k[C12H22O11] (The products of the reaction, glucose and fructose, have the same molecular formulas but differ in the arrangement of the atoms in their molecules.) (a) In neutral solution, k=2.11011s1 at 27 C and 8.51011s1 at 37 C. Determine the activation energy, the frequency factor, and the rate constant for this equation at 47 C (assuming the kinetics remain consistent with the Arrhenius equation at this temperature). (b) When a solution of sucrose with an initial concentration of 0.150 M reaches equilibrium, the concentration of sucrose is 1.65107M . How long will it take the solution to reach equilibrium at 27 C in the absence of a catalyst? Because the concentration of sucrose at equilibrium is so low, assume that the reaction is irreversible. (c) Why does assuming that the reaction is irreversible simplify the calculation in pan (b)?arrow_forwardGaseous azomethane (CH3N2CH3) decomposes to ethane and nitrogen when heated: CH3N2CH3(g) CH3CH3(g) + N2(g) The decomposition of azomethane is a first-order reaction with k = 3.6 104 s1 at 600 K. (a) A sample of gaseous CH3N2CH3 is placed in a flask and heated at 600 K for 150 seconds. What fraction of the initial sample remains after this time? (b) How long must a sample be heated so that 99% of the sample has decomposed?arrow_forwardThe acid-catalyzed iodination of acetone CH3COCH3(aq) + I2(aq) CH3COCH2I(aq) + HI(aq) is a common laboratory experiment used in general chemistry courses to teach the method of initial rates. The reaction is followed spectrophotometrically by the disappearance of the color of iodine in the solution. The following data (J. P. Birk and D. L Walters, Journal of Chemical Education, Vol. 69, p. 585, 1992) were collected at 23 C for this reaction. Determine the rate law for this reaction.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





