
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20.76P
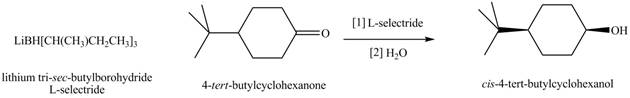
Lithium tri-sec-butylborohydride, also known as L-selectride, is a metal hydride reagent that contains three sec-butyl groups bonded to boron. When this reagent is used to reduce cyclic ketones, one stereoisomer of ten predominates as product. Explain why the reduction of 4-tert-butylcyclohexanone with L-selectride forms the cis alcohol as the major product.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Nerol, a natural product from lemongrass, can be converted to α-cyclogeraniol upon treatment with chlorosulfonic acid(an acid catalyst). Write a stepwise mechanism for this process. The reaction involves a carbocation intermediate.
What is the role of phosphoric acid in the synthesis of cyclohexene?
it is an antioxidant that prevents free radical side reactions
it is a safe, non-toxic solvent
it lowers the boiling point of the reaction mixture (a colligative property of adding
phosphoric acid to water)
it protonates the hydroxyl of cyclohexanol to make it a better leaving group
Synthesize 2-Methyl-4-heptanone from 2-methyl-1-propanol and butanal using the organic or inorganic reagents that are needed
Chapter 20 Solutions
ORGANIC CHEMISTRY
Ch. 20 - Prob. 20.1PCh. 20 - Which carbonyl groups in the anticancer drug taxol...Ch. 20 - Prob. 20.3PCh. 20 - Problem 20.4 What alcohol is formed when each...Ch. 20 - Problem 20.5 What aldehyde or ketone is needed to...Ch. 20 - Prob. 20.6PCh. 20 - Problem 20.7 Draw the products formed when is...Ch. 20 - Problem 20.8 Draw the products formed (including...Ch. 20 - Prob. 20.9PCh. 20 - Problem 20.10 Draw a stepwise mechanism for the...
Ch. 20 - Prob. 20.11PCh. 20 - Problem 20.12 Draw the products formed from ...Ch. 20 - Prob. 20.13PCh. 20 - Prob. 20.14PCh. 20 - Prob. 20.15PCh. 20 - Problem-20.16 Review the oxidation reactions using...Ch. 20 - Problem-20.17 Write the step(s) needed to convert ...Ch. 20 - Problem-20.18 Oct-1-yne reacts rapidly with ,...Ch. 20 - Prob. 20.19PCh. 20 - Prob. 20.20PCh. 20 - Problem 20.21 Draw the product of each reaction.
...Ch. 20 - Problem 20.22 Draw the products (including...Ch. 20 - Problem 20.23 What Grignard reagent and carbonyl...Ch. 20 - Problem 20.24 Linalool (the Chapter 9 opening...Ch. 20 - Problem 20.25 What Grignard reagent and carbonyl...Ch. 20 - Prob. 20.26PCh. 20 - Draw the products formed when each compound is...Ch. 20 - Problem 20.28 What ester and Grignard reagent are...Ch. 20 - Prob. 20.29PCh. 20 - Problem 20.30 What reagent is needed to convert ...Ch. 20 - Prob. 20.31PCh. 20 - What carboxylic acid formed from each alkyl halide...Ch. 20 - Prob. 20.33PCh. 20 - Problem 20.34 Draw the product when each compound...Ch. 20 - Problem 20.35 Synthesize each compound from...Ch. 20 - Prob. 20.36PCh. 20 - 20.37 Devise a synthesis of each alcohol from...Ch. 20 - 20.38 Draw the products formed when pentanal is...Ch. 20 - 20.39 Draw the product formed when is treated...Ch. 20 - The stereochemistry of the products of reduction...Ch. 20 - Prob. 20.41PCh. 20 - 20.42 Draw the products or each reduction...Ch. 20 - Prob. 20.43PCh. 20 - 20.44 Draw all stereoisomers formed in each...Ch. 20 - Prob. 20.45PCh. 20 - 20.46 Treatment of ketone A with ethynylithium...Ch. 20 - 20.47 Explain why metal hydride reduction gives an...Ch. 20 - Prob. 20.48PCh. 20 - 20.49 Identify the lettered compounds in the...Ch. 20 - Prob. 20.50PCh. 20 - 20.51 Draw a stepwise mechanism for the following...Ch. 20 - 20.52 Draw a stepwise mechanism for the following...Ch. 20 - Prob. 20.53PCh. 20 - 20.54 Draw a stepwise mechanism for the following...Ch. 20 - Prob. 20.55PCh. 20 - Prob. 20.56PCh. 20 - 20.57 What ester and Grignard reagent are needed...Ch. 20 - 20.58 What organolithium reagent and carbonyl...Ch. 20 - 20.59 What epoxide and organometallic reagent are...Ch. 20 - Prob. 20.60PCh. 20 - 20.61 Propose two different methods to synthesize...Ch. 20 - 20.62 Synthesize each compound from cyclohexanol...Ch. 20 - 20.63 Convert propan-2-ol into each compound....Ch. 20 - 20.64 Convert benzene into each compound. You may...Ch. 20 - 20.65 Design a synthesis of each compound from...Ch. 20 - 20.66 Synthesize each compound from the given...Ch. 20 - Prob. 20.67PCh. 20 - Prob. 20.68PCh. 20 - 20.69 An unknown compound A (molecular formula )...Ch. 20 - 20.70 Treatment of compound C (molecular formula )...Ch. 20 - 20.71 Treatment of compound E (molecular formula )...Ch. 20 - 20.72 Reaction of butanenitrile () with methyl...Ch. 20 - 20.73 Treatment of isobutene with forms a...Ch. 20 - 20.74 Draw a stepwise mechanism for the following...Ch. 20 - Prob. 20.75PCh. 20 - 20.76 Lithium tri-sec-butylborohydride, also known...Ch. 20 - Prob. 20.77PCh. 20 - Prob. 20.78PCh. 20 - Prob. 20.79PCh. 20 - 20.80 Draw a stepwise mechanism for the following...Ch. 20 - Prob. 20.81P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Wittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forwardIn the chemical synthesis of DNA and RNA, hydroxyl groups are normally converted to triphenylmethyl (trityl) ethers to protect the hydroxyl group from reaction with other reagents. Triphenylmethyl ethers are stable to aqueous base but are rapidly cleaved in aqueous acid. (a) Why are triphenylmethyl ethers so readily hydrolyzed by aqueous acid? (b) How might the structure of the triphenylmethyl group be modified to increase or decrease its acid sensitivity?arrow_forwardAldehydes and ketones react with one molecule of an alcohol to form compounds called hemiacetals, in which there is one hydroxyl group and one ether-like group. Reaction of a hemiacetal with a second molecule of alcohol gives an acetal and a molecule of water. We study this reaction in Chapter 16. Draw structural formulas for the hemiacetal and acetal formed from these reagents. The stoichiometry of each reaction is given in the problem.arrow_forward
- Draw structural formulas for (1) the alkyltriphenylphosphonium salt formed by treatment of each haloalkane with triphenylphosphine, (2) the phosphonium ylide formed by treatment of each phosphonium salt with butyllithium, and (3) the alkene formed by treatment of each phosphonium ylide with acetone.arrow_forwardWhen warmed in dilute sulfuric acid, 1-phenyl-1,2-propanediol undergoes dehydration and rearrangement to give 2-phenylpropanal. (a) Propose a mechanism for this example of a pinacol rearrangement (Section 10.7). (b) Account for the fact that 2-phenylpropanal is formed rather than its constitutional isomer, 1-phenyl-1-propanone.arrow_forwardWhen a primary alcohol is treated with p-toluenesulfonyl chloride at room temperature in the presence of an organic base such as pyridine, a tosylate is formed. When the same reaction is carried out at higher temperature, an alkyl chloride is often formed. Explain.arrow_forward
- Account for the fact that treating propenoic acid (acrylic acid) with HCl gives only 3-chloropropanoic acid.arrow_forwardGeneral Features for Reaction of Organometallic Reagents withAldehydes and Ketones ?arrow_forwardWhen ethyl ether is heated with excess HI for several hours, the only organic product obtained is ethyl iodide. Explain why ethyl alcohol is not obtained as a productarrow_forward
- What reaction converts benzoic acid to m-bromobenzoic acid? Alkylation Acylation Halogenation Hydrohalogenation Hydration Reduction Oxidation Nitration Sulfonationarrow_forwardDescribe how 3-methyl-1-phenyl-3-pentanol can be prepared from benzene. You can use any inorganic reagents and solvents, and any organic reagents provided they contain no more than two carbons.arrow_forwardDraw the principal organic product for the reaction of 1-bromopentane with lithium in diethyl ether, followed by formaldehyde in diethyl ether, and then followed by dilute acid.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY