
Concept explainers
(a)
Interpretation:
The structure of an L- aldopentose needs to be drawn.
Concept introduction:
Sugar molecules can be named as D or L sugars according to their most oxidized carbon at the top of the Fisher projection. Aldopentose is a five-carbon
Answer to Problem 21P
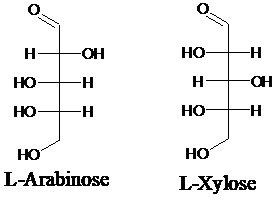
Explanation of Solution
Sugar molecules can be named as D or L sugars according to their most oxidized carbon at the top of the Fisher projection. The absolute configuration of a molecule can be explained using D and L configuration. In D- sugars, the OH group on the bottom chiral center points to the right while in L- sugars, the OH group on the bottom chiral center points to the left. Aldopentose is a five-carbon aldehyde sugar. Xylose, Arabinose and Ribose are few examples.
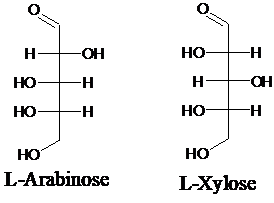
(b)
Interpretation:
The structure of a D- tetrose needs to be drawn.
Concept introduction:
Sugar molecules can name as D or L sugars according to their most oxidized carbon at the top of the Fisher projection. Aldotetrose is a four-carbon aldehyde sugar.
Answer to Problem 21P
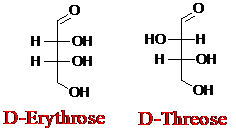
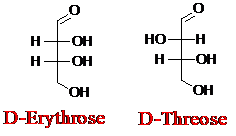
Explanation of Solution
Sugar molecules can be named as D or L sugars according to their most oxidized carbon at the top of the Fisher projection. The absolute configuration of a molecule can be explained using D and L configuration. In D- sugars, the OH group on the bottom chiral center points to the right while if L-sugars, the OH group on the bottom chiral center points to the left. Aldotetrose is a four-carbon aldehyde sugar.
(c)
Interpretation:
The structure of a five-carbon alditol needs to be drawn.
Concept introduction:
Alditols are polyols. Alditols can be produced by reducing an aldehyde or a
Answer to Problem 21P
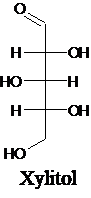
Explanation of Solution
Alditols are polyols. Alditols can be produced by reducing an aldehyde or a ketone group in a monosaccharide. In this reduction process, an aldehyde group or a ketone group converts into −CH2OH group. Xylitol is an example of an alditol.

For an example:
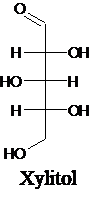
Want to see more full solutions like this?
Chapter 20 Solutions
GENERAL,ORGANIC,+BIOLOG.CHEM. (LOOSE)
- Fructose, present with glucose in honey, reacts with Benedicts reagent. Circle the structural features that enable fructose to react.arrow_forwardA monosaccharide that consists of 4 carbon atoms, one of which is part of a aldehyde functional group, is classified as a(n) a). Aldotetrose b). Aldohexose c). Ketotetrose d). Ketohexosearrow_forwardBenedict’s solution can be used to distinguish between: a) fructose and glucose b) maltose and lactose c) maltose and amylose d) amylose and amylopectinarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





