
Concept explainers
(a)
Interpretation:
The following compound must be labeled as a monosaccharide, aldonic acid or an alditol:
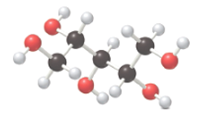
Concept introduction:
Monosaccharides are also called simple sugars, contain
(b)
Interpretation:
The following compound must be labeled as a monosaccharide, aldonic acid or an alditol:
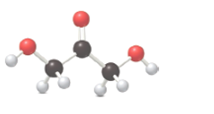
Concept introduction:
Monosaccharides are also called simple sugars, contain aldehyde or ketone groups and many hydroxyl groups. Aldonic acids contain many hydroxyl groups and an acid group. Alditol are polyols which contain many hydroxyl groups.
(c)
Interpretation:
The following compound must be labeled as a monosaccharide, aldonic acid or an alditol:
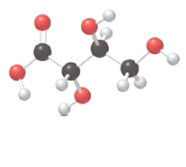
Concept introduction:
Monosaccharides are also called simple sugars, contain aldehyde or ketone groups and many hydroxyl groups. Aldonic acids contain many hydroxyl groups and an acid group. Alditol are polyols which contain many hydroxyl groups.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
- a) Draw Haworth projections of both - and -anomers of D-fructose. Indicate which carbon is the anomeric carbon.b) Sucrose is a disaccharide made up of a molecule of D-fructose and D-glucose. Draw the structure of sucrose clearly indicating the linkage between the two monosaccharides and its biological significance.c) Tollen’s reagent is a very mild oxidizing agent which normally oxidize aldehydes but not ketones. However, both glucose and fructose give positive results with Tollen’s reagent and are classified as reducing sugars. Explain how fructose can also give positive results with Tollen’s reagent (illustrate using structures).arrow_forward(a) Convert each cyclic monosaccharide into a Fischer projection of its acyclic form. (b) Name each monosaccharide. (c) Label the anomer as a or ß.arrow_forwardSOURCE: GENERAL ORGANIC AND BIOLOGICAL CHEMISTRY BY SMITH 4TH EDITIONarrow_forward
- Identify a polysaccharide that is related to each statement below. a. Makes up about 80% of starch and contains branching b. Not digestible by humans, contains beta glycosidic bonds c. Animal starch, stored in liver and musclesarrow_forward- ----- -- --- . 20.45 What monosaccharides are formed when the following disaccharide is hydrolyzed? CH2OH CH,OH H. H. H O. H H. ОН НО O. OH OH OH OH H.arrow_forwardA monosaccharide that consists of 4 carbon atoms, one of which is part of a aldehyde functional group, is classified as a(n) a). Aldotetrose b). Aldohexose c). Ketotetrose d). Ketohexosearrow_forward
- For the following trisaccharide: a. Identify its monosaccharide units, specifying the anomeric forms of of each unit present on the trisaccharide. b. What are the glycosidic bonds present on the trisaccharide? c. Is the trisaccharide a reducing sugar?arrow_forwardWhat monosaccharides are formed in a modified Kiliani–Fischer synthesis starting with each of the following monosaccharides? a. D-xylose b. L-threosearrow_forwardB. Complete the following chart: (Disaccharides) Disaccharide Monosaccharide Subunits Natural Sourcearrow_forward
- Which monosaccharide is a-glucose? CH,OH CH,OH CH;OH CH,OH H. H. OH H. OH HO. H. OH H. H. H. HO. H. OH H. H. H. OH H. H. H. OH H. H. H. H. H. C OD O B O C OA A,arrow_forwardStructures of monosaccharides are a) polymers b) pyranoses or furanoses c) aldoses or ketoses d) B and Carrow_forwardwhat type of saccharide is this molecule?arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning  General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





