
Interpretation:
Difference between eluent and eluate has to be given.
Concept Introduction:
Principle of chromatography is the mobility of analyte across two phase namely mobile and stationary phase. For effective separation of analyte, the sample may be required to dissolve in some gas or liquid /supercritical fluids referred to as mobile phases. Another phase used in the chromatographic analysis is the stationary phase often fixed as inert silica gel or as liquid silicon grease adhered to an inert solid support. Immiscible phase has got adsorption sites to retain analyte as it runs along with the mobile phase.
Explanation of Solution
Phenomenon that allows analyte injected within a chromatographic column to get separated when the mobile phase runs over a stationary solid phase is known as elution.
Two fluids, eluent, and eluate can be distinguished in the illustration as follows:
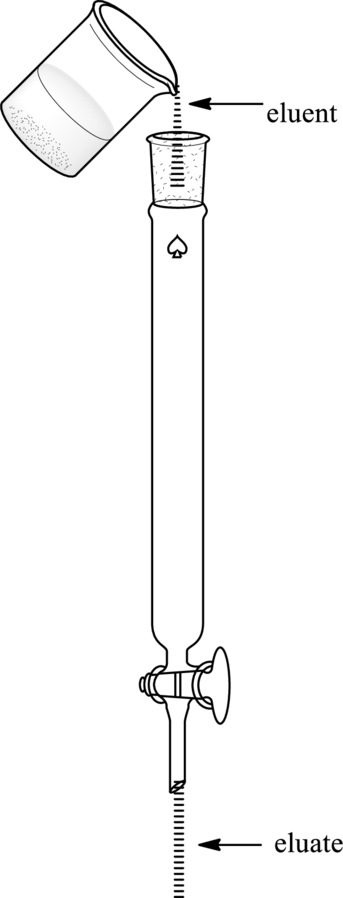
Hence, eluent may be defined as a fluid that enters from the top of the chromatographic column while fluid that is released from the other end is termed as an eluate.
Want to see more full solutions like this?
Chapter 21 Solutions
Exploring Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





