
Concept explainers
(a)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
The saponification reaction is the hydrolysis reaction of ester. Ester undergoes hydrolysis in presence of base in aqueous solution. The ester upon hydrolysis forms the carboxylate salt and alcohol. The presence of
Answer to Problem 21.54AP
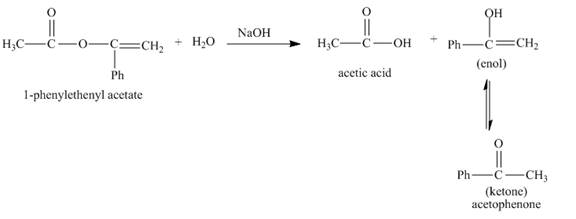
The complete given reaction with the product formed is shown below.
Explanation of Solution
The given incomplete reaction is shown below.
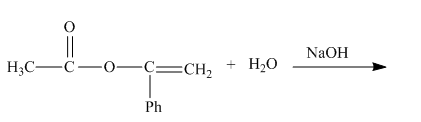
Figure 1
In the given reaction, the hydrolysis of ester takes place in the presence of base. The hydroxide ion of the sodium hydroxide reacts with the carbonyl carbon of the ester. The hysroxide group is a nucleophile which attacks at the electrophilic carbon. The hydrolysis of the ester gives acetic acid and enol. The enol tautomerises to form keto form. Both keto-enol co-exists in equilibrium. The complete reaction is shown below.
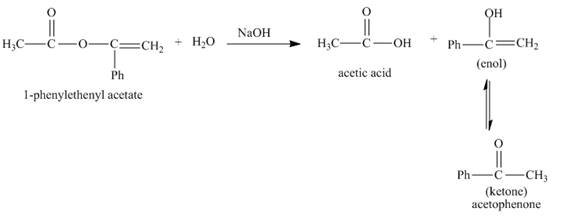
Figure 2
The complete given reaction with the products formed is shown in Figure 2.
(b)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
Trans esterification reaction is the reaction in which
Answer to Problem 21.54AP
The complete given reaction is shown below.
Explanation of Solution
The given incomplete reaction is shown below.
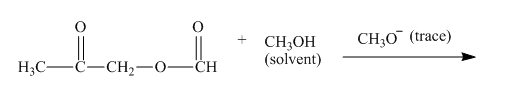
Figure 3
The
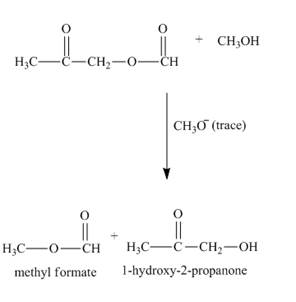
Figure 4
The complete reaction along with products formed is shown in Figure 4.
(c)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
Reaction of acid chloride with amine form amide compound. The phosgene reacts in similar way that of acid chloride with
Answer to Problem 21.54AP
The complete given reaction is shown below.
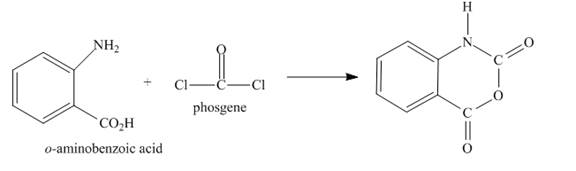
Explanation of Solution
The given incomplete reaction is shown below.
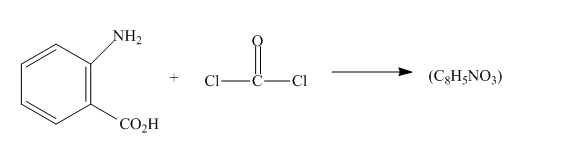
Figure 5
The lone pair of nitrogen of amine group attacks at the electrophilic carbonyl carbon of the phosgene. The chloride ion acts as leaving group and amide bond is formed. The amino group of

Figure 6
The complete reaction along with products formed is shown in Figure 6.
(d)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
Amide is formed in the reaction between acid chloride and amine. Hydrazine also reacts in same way. The hydrazine contains two amine groups, so it will reacts with the two equivalents of the acid chloride. Both the amine group of hydrazine gets acylated upon reaction with excess benzoyl chloride.
Answer to Problem 21.54AP
The complete given reaction is shown below.
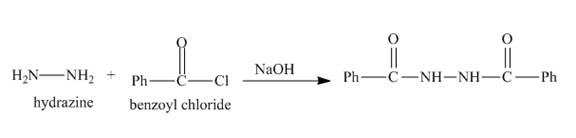
Explanation of Solution
The given incomplete reaction is shown below.
Figure 7
The nitrogen atom of hydrazine acts as nucleophile and attacks at the carbonyl carbon of benzoyl chloride. It results in the formation of amide linkage. As, hydrazine posses two amine groups so the other amine group will also reacts with benzoyl chloride in the same way to form the desired product. The complete reaction is shown below.
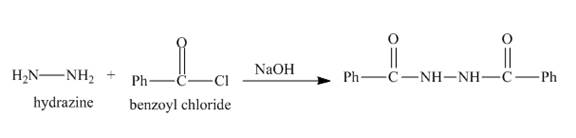
Figure 8
The complete reaction along with products formed is shown in Figure 8.
(e)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
Amide group undergoes hydrolysis reaction in presence of an acid to form carboxylic acid and an amine. The nitrile group undergoes hydrolysis to form carboxylic acid and ammonia. The hydrolysis of nitrile takes place in strongly acidic medium.
Answer to Problem 21.54AP
The complete given reaction is shown below.
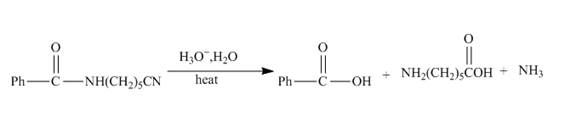
Explanation of Solution
The incomplete reaction is shown below.
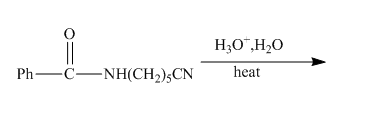
Figure 9
The hydrolysis of the amide bond takes place in acid or basic medium. The given compound in the presence of an acid will gert hydrolyzed. The hydrolysis of amide group forms amine and carboxylic acid. The hydrolysis of the nitrile group also takes place in strongly acidic medium to form carboxylic acid and ammonia. The given compound contains both amide and cyanide group, so both groups will get hydrolyzed. The resulting products will be acid, amino acid and ammonia. The complete reaction is shown below.

Figure 10
The complete reaction along with products formed is shown in Figure 10.
(f)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
Lactam is a cyclic amide compound. It will undergoes reduction reaction to form cyclic amine compounds. The reduction of lactam does not results in ring opening. Lithium aluminium hydride is used as the reducing agent. Imine intermediate is formed in the reduction of lactam.
Answer to Problem 21.54AP
The complete given reaction is shown below.
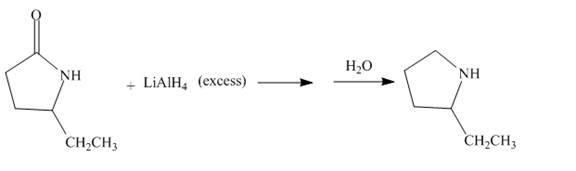
Explanation of Solution
The given incomplete reaction is shown below.
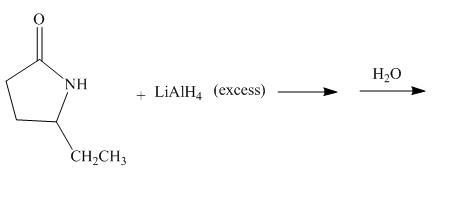
Figure 11
The lithium aluminium hydride is a strong reducing agent. It contains hydride ion which reacts with the carbonyl carbon. It will reduce the amide group to the amine group. The given compound is a lactam. The lactam will get reduced to cyclic amine. The reaction proceeds via cyclic imine intermediate. Cyclic imine intermediate is further reduced to the cyclic amine compound. The complete reaction is shown below.

Figure 12
The complete reaction along with products formed is shown in Figure 12.
(g)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
Grignard reagent reacts with esters to form tertiary alcohol. The reaction of lactone with grignard reagent also proceeds via same mechanism. The lactone is a cyclic ester compound. Lactone reacts with grignard reagent to form the dialcohol compound.
Answer to Problem 21.54AP
The complete given reaction is shown below.
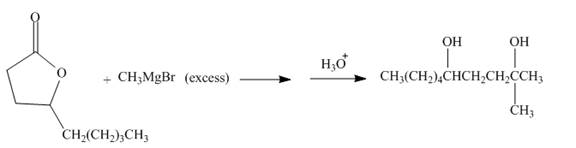
Explanation of Solution
The given incomplete reaction is shown below.
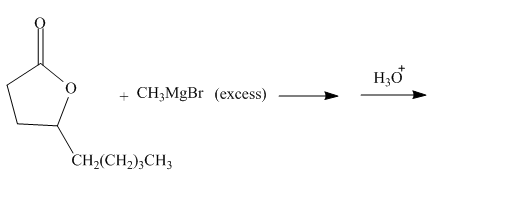
Figure 13
The carbonyl carbon of the lactone will undergo nucleophilic addition reaction with methyl magnesium bromide. The lactone will be coverted into the tertiary alcohol compound. Two equivalents of methyl magnesium bromide will react with the lactone to form the dialcohol product. The complete reaction is shown below.

Figure 14
The complete reaction along with products formed is shown in Figure 14.
(h)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
Grignard reagent reacts with esters to form tertiary alcohol. The reaction involves acyl substitution reaction which is followed by nucleophilic addition reaction. The reaction alkyl group of grignard reagent acts as the nucleophile and attacks at the carbonyl carbon of ester.
Answer to Problem 21.54AP
The complete given reaction is shown below.
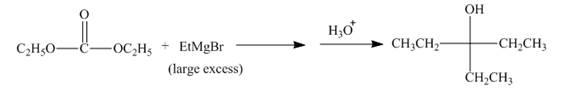
Explanation of Solution
The given incomplete reaction is shown below.
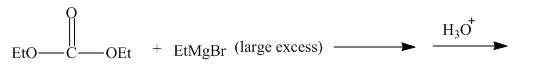
Figure 15
The ethyl group of the ethyl magnesium bromide acts as nucleophile and attacks at the carbonyl carbon of the ester, which resuults in the formation of an ethyl ester with the removal of one
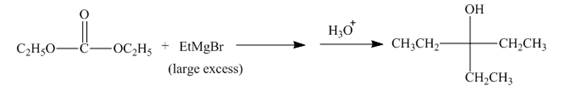
Figure 16
The complete reaction along with products formed is shown in Figure 16.
(i)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
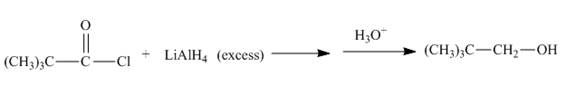
Acid chloride reacts with lithium aluminium hydride to form alcohols. Lithium aluminium hydride is a strong reducing agent. The conversion of acid chloride into
Answer to Problem 21.54AP
The complete given reaction is shown below.
Explanation of Solution
The given incomplete reaction is shown below.
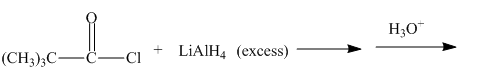
Figure 17

Figure 18
The complete reaction along with products formed is shown in Figure 18.
(j)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
Grignard reagent reacts with esters to form tertiary alcohol. The reaction involves acyl substitution reaction which is followed by nucleophilic addition reaction. The reaction alkyl group of grignard reagent acts as the nucleophile and attacks at the carbonyl carbon of ester.
Answer to Problem 21.54AP
The complete given reaction is shown below.
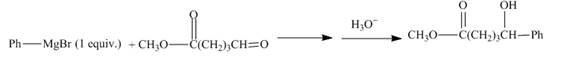
Explanation of Solution
The given incomplete reaction is shown below.
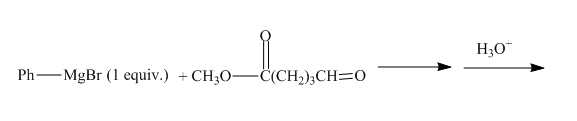
Figure 19
The given compound contains both the ester and aldehyde group. The grignard reagent reacts with both the groups, but if grignard reagent is taken in small amount then it will react with only the more reactive functional group. Aldehyde group is more reactive than the ester group so it will react with the grignard reagent. The grignard reagent will convert the aldehyde group to the alcohol group. The complete reaction is shown below.

Figure 20
The complete reaction along with products formed is shown in Figure 20.
(k)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
Trans esterification reaction is the reaction in which
Answer to Problem 21.54AP
The complete given reaction is shown below.
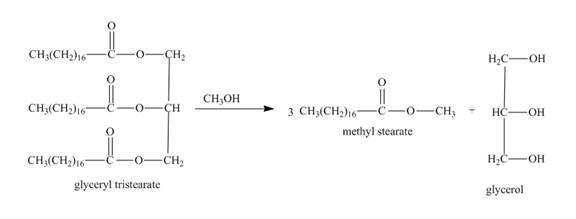
Explanation of Solution
The given incomplete reaction is shown below.
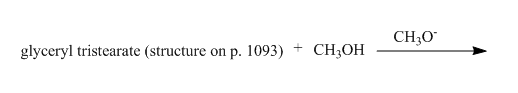
Figure 21
In this the glyceryl tristearate is a tri ester compound. In the presence of the methanol it will undergo transesterification reaction. The glyceryl tristearate reacts with methanol to form three equivalents of methyl stearate and one equivalent of glycerol. The complete reaction is shown below.

Figure 22
The complete reaction along with products formed is shown in Figure 22.
(l)
Interpretation:
The given reaction is to be completed and product is to be shown.
Concept Introduction:
Lactam are cyclic amide compound. It formed by the nucleophilic substitution reaction between the amine group and ester group present in the same molecule. Lactam undergoes reduction reaction to form cyclic amines. The nitrogen of amine group acts as nucleophilie and carbonyl carbon of ester acts as electriphilic centre.
Answer to Problem 21.54AP
The complete given reaction is shown below.
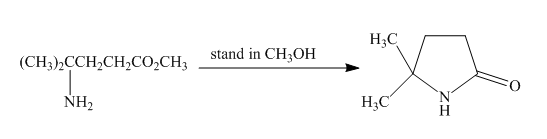
Explanation of Solution
The given incomplete reaction is shown below.
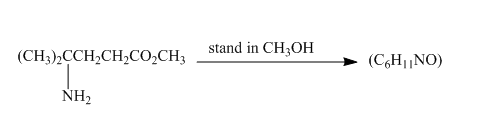
Figure 23
Esters on reaction with amine form amide compounds. As in the given compound the amine group and the ester group are present in the same molecule so upon reaction they will form cyclic amide compound known as lactam. The lone pair of nitogen atom attacks the carbonyl carbon of the ester to form the cyclic lactam with the elimination of alkoxy group. The complete reaction is shown below.

Figure 24
The complete reaction along with products formed is shown in Figure 24.
Want to see more full solutions like this?
Chapter 21 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Outline the synthesis of the following from either malonic ester or acetoacetic ester or an enamine and using any other reagents of your choice:arrow_forwardGive a clear handwritten answer with explanation needed...give the mechanism of given bleow reactionarrow_forwardExplain clearly Giving and example and mechanisms, explain the aromatic Substitution reactions.arrow_forward
- the following reaction scheme leads to the formation of compound B. give the structure of the final products and of the intermediate product A and justify, using the mechanism, the formation of thesearrow_forwardGive the structural chemistry of active methylene, classify it asradical/intermediate/stable organic compound, reagent of its production and role in alkylationarrow_forwardA synthesis of the pain reliever phenacetin is outlined in the following equation. What is the structure of phenacetin?arrow_forward
- Based on the theoretical discussion, illustrate with equations: a. How ethers are broken down by zinc chloridearrow_forwardIf you were ask to prepare ethylamine, suggest two ways which you would give that would give you exclusively the desired product. Explain what is happening in each reaction steps.arrow_forwardRank the basicity of the following sets of compounds. And give explanation.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
