
Concept explainers
(a)
Interpretation:
The product obtained from the reaction of ethyl benzoate and
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
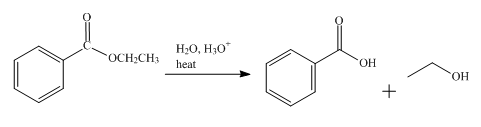
The product obtained from the reaction of ethyl benzoate and
Explanation of Solution
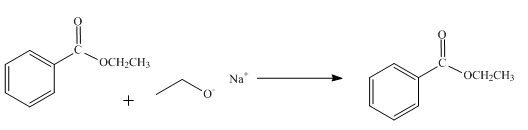
The reaction of ethyl benzoate in presence

Figure 1
The product obtained from the reaction of ethyl benzoate and
(b)
Interpretation:
The product obtained from the reaction of ethyl benzoate and
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
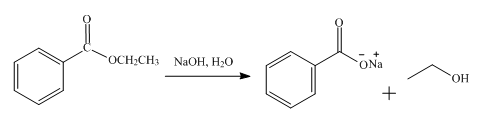
The product obtained from the reaction of ethyl benzoate and
Explanation of Solution
The reaction of ethyl benzoate in the presence of

Figure 2
The product obtained from the reaction of ethyl benzoate and
(c)
Interpretation:
The product obtained from the reaction of ethyl benzoate and aqueous
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
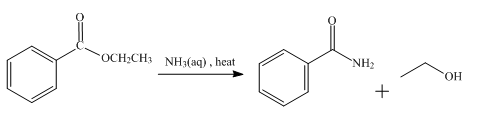
The product obtained from the reaction of ethyl benzoate and aqueous
Explanation of Solution
The reaction of ethyl benzoate in the presence of aqueous

Figure 3
The product obtained from the reaction of ethyl benzoate and aqueous
(d)
Interpretation:
The product obtained from the reaction of ethyl benzoate and
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
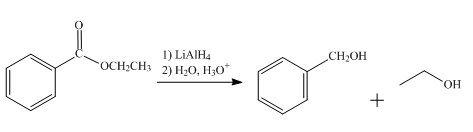
The product obtained from the reaction of ethyl benzoate and
Explanation of Solution
The reduction reaction of ethyl benzoate takes place in the presence of

Figure 4
The product obtained from the reaction of ethyl benzoate and
(e)
Interpretation:
The product obtained from the reaction of ethyl benzoate and excess
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
The product obtained from the reaction of ethyl benzoate and excess
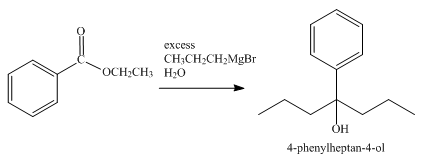
Explanation of Solution
The reaction of ethyl benzoate with an excess of Grignard reagent

Figure 5
The product obtained from the reaction of ethyl benzoate and excess
(f)
Interpretation:
The product obtained from the reaction of the product of part (e) and acetyl chloride, pyridine at
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
The product obtained from the reaction of the product of part (e) and acetyl chloride, pyridine, at
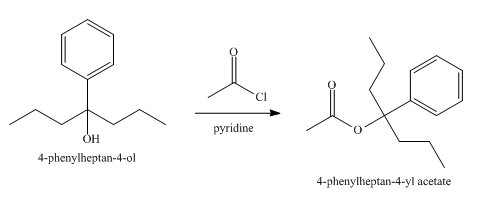
Explanation of Solution
The reaction of the product of part (e) and acetyl chloride, pyridine at

Figure 6
The product obtained from the reaction of the product of part (e) and acetyl chloride, pyridine, at
(g)
Interpretation:
The product obtained from the reaction of the product of part (e) and benzenesulfonyl chloride is to be stated.
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
The product obtained from the reaction of the product of part (e) and benzenesulfonyl chloride is shown below.
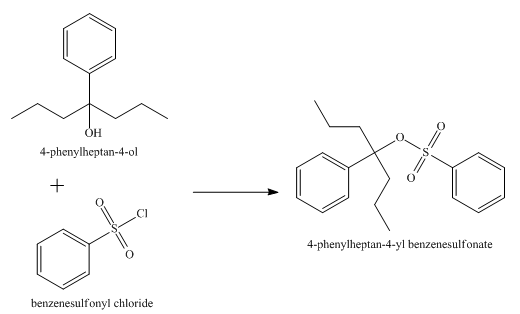
Explanation of Solution
The reaction of the product of part (e) and benzenesulphonyl chloride undergoes substitution reaction. It results in the formation of

Figure 7
The product obtained from the reaction of the product of part (e) and benzenesulfonyl chloride is shown in Figure 7.
(h)
Interpretation:
The product obtained from the reaction of ethyl benzoate and
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 21.32AP
The product obtained from the reaction of ethyl benzoate and
Explanation of Solution
The reaction of ethyl benzoate with

Figure 8
The product obtained from the reaction of ethyl benzoate and
Want to see more full solutions like this?
Chapter 21 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- In an advanced synthetic chemistry experiment, a researcher prepares a compound, ZY-7, by reacting a ketone (C5H10O) with hydroxylamine (NH2OH), followed by heating in the presence of an acid catalyst. The resulting compound, ZY-7, is then treated with a solution of sodium nitrite (NaNO2) and hydrochloric acid (HCl) at low temperature. Identify the class of compound that ZY-7 most likely belongs to after this series of reactions." A) Amide B) Oxime C) Nitro compound D) Diazonium salt E) Ester Don't use chatgpt please provide valuable answerarrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst to give hydrocarbon B. Hydrocarbon A also reacts with OsO4 to give the glycol C. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2COOH, and the other fragment is ketone D (R2C=O). What are the structures of A, B, C and D? Write all reactions.arrow_forwardThe compound acetophenone has a very similar molar mass to that of benzoic acid and benzamide. However, acetophenone has a much lower m.p. (20 °C) than both such that, by contrast, it is a liquid at room temperature. By considering intermolecular forces and comparing functional group structure, account for this big difference in physical properties.arrow_forward
- An unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forwardBenzoic acid, Ph-COOH (C6H5CO2H), is not soluble in water while it dissolves in ether (diethyl ether), (CH3CH2)2O. Yet upon treatment with sodium hydroxide, benzoic acid turns hydrophilic and dissolves in water. Provide chemical explanation of this observation.arrow_forwardAn organic compound A of unknown structure was found to have a molecular formula C8H16. When A was poured in water and heated, compound B having a molecular formula C8H18O was formed. B upon heating with sulfuric acid was converted to C as the major product which is identical to A. Ozonolysis of C gave one molecule each of two different products D and E, both having a molecular formula C4H8O. Write the reactions involved and determine the structure of A,B,C,D and E.arrow_forward
- Three constitutional isomers of molecular formula C 5H 8O can be converted to 1-pentanol (CH 3CH 2CH 2CH 2CH 2OH) on treatment with two equivalents of H 2 in the presence of a Pd catalyst. Draw the structures of the three possible compounds, all of which contain a carbonyl grouparrow_forwardGive the expected organic product when phenylacetic acid, PhCH2COOH, is treated with reagent Q.)NaOH, H2Oarrow_forwardGive the expected organic product when phenylacetic acid, PhCH2COOH, is treated with reagent Q.)NaHCO3, H2Oarrow_forward
- Explain why methyl trifluoroacetate, CF3CO2CH3, is more reactive than methyl acetate, CH3CO2CH3, in nucleophilic acyl substitution reactions.arrow_forwardQuinolines, heterocyclic compounds that contain a pyridine ring fused to a benzene ring, are commonly synthesized by a method known as the Skraupsynthesis, in which aniline reacts with glycerol under acidic conditions. Nitrobenzene is added to the reaction mixture to serve as an oxidizing agent.The first step in the synthesis is the dehydration of glycerol to propenal. a. What product would be obtained if para-ethylaniline were used instead of aniline?b. What product would be obtained if 3-hexen-2-one were used instead of glycerol?c. What starting materials are needed for the synthesis of 2,7-diethyl-3-methylquinoline?arrow_forwardQuinolines, heterocyclic compounds that contain a pyridine ring fused to a benzene ring, are commonly synthesized by a method known as the Skraup synthesis, in which aniline reacts with glycerol under acidic conditions. Nitrobenzene is added to the reaction mixture to serve as an oxidizing agent. The first step in the synthesis is the dehydration of glycerol to propenal. a. What product would be obtained if para-ethylaniline were used instead of aniline? b. What product would be obtained if 3-hexen-2-one were used instead of glycerol? c. What starting materials are needed for the synthesis of 2,7-diethyl-3-methylquinoline?arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


