
Quantitative Chemical Analysis
9th Edition
ISBN: 9781464135385
Author: Daniel C. Harris
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Chapter 21, Problem 21.AE
Interpretation Introduction
Interpretation:
A standard addition graph has to be prepared to find the concentration of
Expert Solution & Answer
Explanation of Solution
The
Graph, that has intensity against the concentration of standard that is added has x intercept of
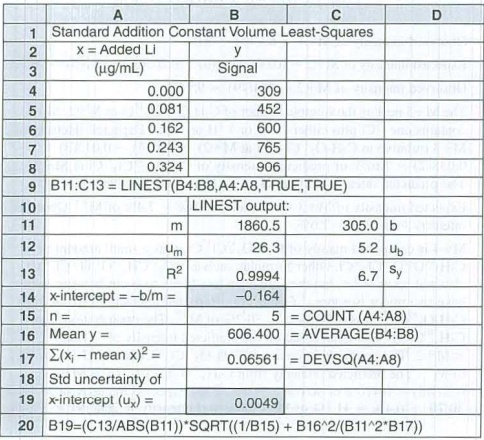
Figure 1
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Chapter 21 Solutions
Quantitative Chemical Analysis
Ch. 21 - Prob. 21.AECh. 21 - Prob. 21.BECh. 21 - Prob. 21.CECh. 21 - Prob. 21.DECh. 21 - Prob. 21.EECh. 21 - Prob. 21.1PCh. 21 - Prob. 21.2PCh. 21 - Prob. 21.3PCh. 21 - Prob. 21.4PCh. 21 - Prob. 21.5P
Ch. 21 - Prob. 21.6PCh. 21 - Prob. 21.7PCh. 21 - Prob. 21.8PCh. 21 - Prob. 21.9PCh. 21 - Prob. 21.10PCh. 21 - Prob. 21.11PCh. 21 - Prob. 21.12PCh. 21 - Prob. 21.13PCh. 21 - Prob. 21.14PCh. 21 - Prob. 21.15PCh. 21 - Prob. 21.16PCh. 21 - Prob. 21.17PCh. 21 - Prob. 21.18PCh. 21 - Prob. 21.19PCh. 21 - Prob. 21.20PCh. 21 - Prob. 21.21PCh. 21 - Prob. 21.22PCh. 21 - Prob. 21.23PCh. 21 - Prob. 21.24PCh. 21 - Prob. 21.25PCh. 21 - Prob. 21.26PCh. 21 - Prob. 21.27PCh. 21 - Prob. 21.28PCh. 21 - Prob. 21.29PCh. 21 - Prob. 21.30PCh. 21 - Prob. 21.31P
Knowledge Booster
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY