
Concept explainers
The Enolate Chemistry of Dianionss
The synthetic applications of carbanions as reagents for carbon
Consider acetic acid:
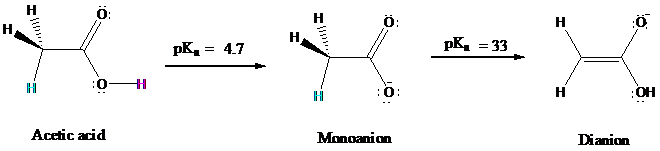
The pKa of acetic acid is
for ionization of a
is shared by carbon. The dianion, however, has carbanionic character and the potential to act as a
nucleophile in carbon
Diisopropylamine has a pKa of
enough base to convert acetic acid to its dianion. Other carboxylic acids behave similarly to give
dianions that undergo typical carbanion reactions. Alkylation of carboxylic acid dianions provide a useful alternative to the malonic ester synthesis.
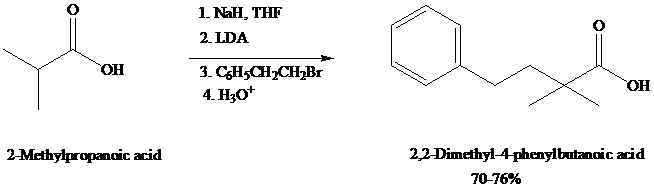
Experimentally, as in this example, it is sometimes useful to convert the carboxylic acid to its carboxylate (monoanion) with sodium hydride (NaH, step
The dianions of α-halocarboxylic acids give epoxy acids (called glycidic acids) on reaction with
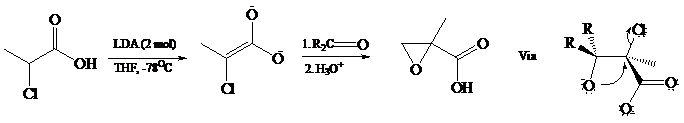
Dianions have been prepared from
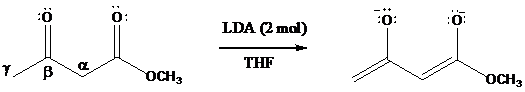
Protons on the
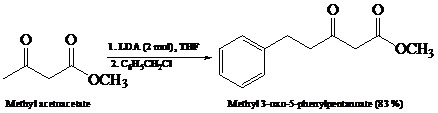
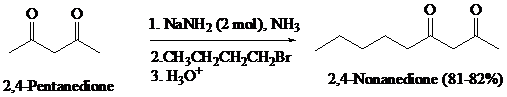
What is the product of the reaction of the dianion in Problem
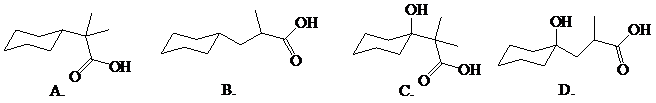
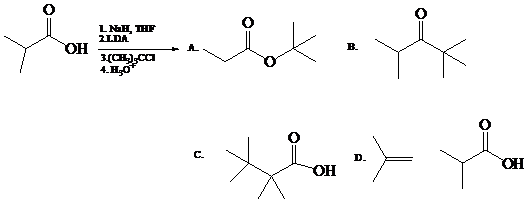
Predict the major organic product(s) of the following reaction
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Connect Access Card Two Year for Organic Chemistry
- The molecular formula of unknown compound B is C10H16.Quantitative brominationof a 0.473-g sample of compound Brequired 48.22mL of a 0.216MBr2/CCl4to produce a color change. How many rings and pi bonds does compound Bcontain?Include any explanations/calculations to justify your answers.arrow_forwardProvide a reasonable synthetic strategy for the synthesis of a racemic mixture of (1R,2R) and (1S,2S)-2-bromo-1-methylcyclopentanol from the compound shown below... Provide the bond line structure for the major organic products obtained in each step of the proposed strategyarrow_forwardGive a clear handwritten answer with explanation..complete the following reactions with their stereochemistry?arrow_forward
- For each of the following, write the major product(s) and then draw out each step in the mechanism using curved arrows. Show ALL lone pair electrons and formal charges. Redraw ALL molecules as to show explicitly ALL bonds being broken or formed. Identify the molecular orbital (HOMO) of the nucleophile and the molecular orbital (LUMO) of electrophile involved in the nucleophilic attack. MO diagrams are not necessary..arrow_forwardKepone, aldrin, and chlordane are synthesized from hexachlorocyclopentadiene and other five-membered-ring compounds. Show how these three pesticides are composed of two five-membered rings.arrow_forwardGive the structures of two different alkyl bromides both of which yield the indicated alkene as the exclusive product of E2 elimination.arrow_forward
- Give the structure of a, b, c, d and e in clear handwritten?arrow_forward3c)Referring to the intermediates you drew in problem below explain in detail why no meta product is obtained in the Friedel-Crafts alkylation of chlorobenzene. Draw all pertinent resonance structures to support your argument.arrow_forwardRank these nucleophiles from most to least reactive for substitution reactions and give a brief rationale for your answer.arrow_forward
- Give the structure of the main product of each of the following Diels-Alder reactions. Make sureto show the stereochemistry of the products when unambiguous.arrow_forwardPlease provide a curved-arrow mechanism for the following reaction. Be sure to use your mechanism and a few words to explain the regiochemistry of the bromines in the final product and why each carbocation intermediate in your mechanism is the preferred intermediate for that step. Include ALL lone pairs and formal charges. Thank you.arrow_forwardHighlight the allylic hydrogen(s) in this structure.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

