
Connect Access Card Two Year for Organic Chemistry
10th Edition
ISBN: 9781259636868
Author: Francis Carey
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 21.1, Problem 3P
Interpretation Introduction
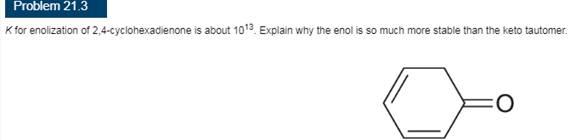
Interpretation:
The explanation corresponding to the fact that the enol form of
Concept Introduction:
In keto-enol tautomerism, the hydrogen atom that is connected to the
Enol refers to an intermediate structure that consists of an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Prepare each compound from cyclopentanol. More than one step may beneeded.
Devise a synthesis of dodec-7-yn-5-ol from hex-1-ene(CH3CH2CH2CH2CH=CH2) as the only organic starting material. You mayuse any other needed reagents.
(a) What is the structure of C, which is formed by oxy-Cope rearrangement of B with NaOEt? (b) Draw a stepwise mechanism for the conversion of C to the bicyclic alcohol D.
Chapter 21 Solutions
Connect Access Card Two Year for Organic Chemistry
Ch. 21.1 - Prob. 1PCh. 21.1 - Prob. 2PCh. 21.1 - Prob. 3PCh. 21.1 - Prob. 4PCh. 21.1 - Prob. 5PCh. 21.2 - Prob. 6PCh. 21.2 - Prob. 7PCh. 21.2 - Prob. 8PCh. 21.3 - Prob. 9PCh. 21.3 - Prob. 10P
Ch. 21.3 - Prob. 11PCh. 21.4 - Prob. 12PCh. 21.4 - Prob. 13PCh. 21.5 - Prob. 14PCh. 21.5 - Problem 21.15 Write the structure of the Dieckmann...Ch. 21.5 - Prob. 16PCh. 21.5 - Prob. 17PCh. 21.6 - Prob. 18PCh. 21.6 - Prob. 19PCh. 21.6 - Prob. 20PCh. 21.6 - Prob. 21PCh. 21.6 - Prob. 22PCh. 21.7 - Prob. 23PCh. 21.8 - Problem 21.24 Mesityl oxide is an industrial...Ch. 21.8 - Prob. 25PCh. 21.8 - Prob. 26PCh. 21.8 - Prob. 27PCh. 21.8 - Prob. 28PCh. 21 - Prob. 29PCh. 21 - Terreic acid, a naturally occurring antibiotic...Ch. 21 - Prob. 31PCh. 21 - Prob. 32PCh. 21 - Prob. 33PCh. 21 - Prob. 34PCh. 21 - Give the structure of the expected organic product...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - Prob. 38PCh. 21 - Prob. 39PCh. 21 - Give the structure of the principal organic...Ch. 21 - Prob. 41PCh. 21 - Prob. 42PCh. 21 - Prob. 43PCh. 21 - Prob. 44PCh. 21 - Prob. 45PCh. 21 - Prob. 46PCh. 21 - Prob. 47PCh. 21 - The use of epoxides as alkylating agents for...Ch. 21 - Prob. 49PCh. 21 - Show how you could prepare each of the following...Ch. 21 - Prob. 51PCh. 21 - Prob. 52PCh. 21 - Prob. 53PCh. 21 - Prob. 54PCh. 21 - The - methylene ketone sarkomycin has an...Ch. 21 - - Lactone can be prepared in good yield from...Ch. 21 - Prob. 57PCh. 21 - Prob. 58DSPCh. 21 - The Enolate Chemistry of Dianionss The synthetic...Ch. 21 - Prob. 60DSPCh. 21 - Prob. 61DSPCh. 21 - Prob. 62DSPCh. 21 - Prob. 63DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ochem question about reagents For the following syntheses what reagents would be needed?arrow_forward(a) What is the structure of C, which is formed by oxy-Coperearrangement of B with NaOEt? (b) Draw a stepwise mechanism for theconversion of C to the bicyclic alcohol D.arrow_forwardAn allylic alcohol contains an OH group on a carbon atom adjacent to aC—C double bond. Treatment of allylic alcohol A with HCl forms amixture of two allylic chlorides, B and C. Draw a stepwise mechanismthat illustrates how both products are formed.arrow_forward
- organic chemistry 32) How many monochlorination product(s) is obtained by chlorination of 2-methylpropane with Cla/ UV light?arrow_forwardQ: devise an efficient method for preparing m cresol from m toluidinearrow_forwardFlexibilene is a terpene isolated from Sinularia flexibilis, a soft coralfound in the Indian Ocean. Draw a stepwise mechanism for the formationof flexibilene from farnesyl diphosphate and isopentenyl diphosphate.What is unusual about the cyclization that forms the 15-membered ringof flexibilene?arrow_forward
- β-Vetivone is isolated from vetiver, a perennial grass that yields a variety of compounds used in traditional eastern medicine, pest control, and fragrance. In one synthesis, ketone A is converted to β-vetivone by a two-step process:Michael reaction, followed by intramolecular aldol reaction. (a) What Michael acceptor is needed for the conjugate addition? (See Problem 23.61 for another method to form the bicyclic ring system of β-vetivone.) (b) Draw a stepwise mechanism for the aldol reaction, which forms the six-membered ring.arrow_forwardWhena nucleophiles attack the carbonyl carbon in an acid the final product is an alcohol. but when a weaker nucleophile is used the carbon oxygen double bond reforms T/F? Group of answer carrow_forwardWhy does the alpha hydrogen to a ketone have a lower pka value than the alpha h to an alkene. Draw a picture to explain ur a waysarrow_forward
- (R)-Carvone, the major component of the oil of spearmint, undergoesacid-catalyzed isomerization to carvacrol, a major component of the oilof thyme. Draw a stepwise mechanism and explain why thisisomerization occurs.arrow_forwardBromoetherification, the addition of the elements of Br and OR to adouble bond, is a common method for constructing rings containingoxygen atoms. This reaction has been used in the synthesis of thepolyether antibiotic monensin (Problem 18.34). Draw a stepwisemechanism for the following intramolecular bromoetherification reaction.arrow_forwardDevise a synthesis of the following compound from styrene. You may use any inorganic or organic reagents. More than one step is required. C6H5CH(OH)CH2C≡CHarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning