
Concept explainers
(a)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized using aldol condensation in good yield. The starting materials are
Explanation of Solution
The given compound is shown below.
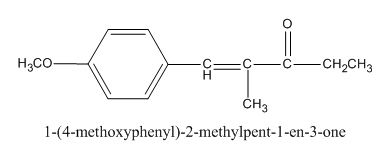
Figure 1
In the above preparation of the compound, the enolate is generated on the
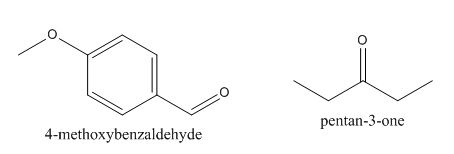
Figure 2
The given compound can be synthesized by aldol condensation of
(b)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized using aldol condensation in good yield. The starting materials are benzaldehyde and
Explanation of Solution
The given compound is shown below.
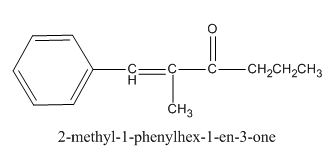
Figure 3
In the preparation of the above compound, the enolate will be generated on the
The structures of the starting materials which are used to produce the given compound are shown below.
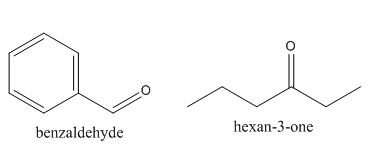
Figure 4
The given compound can be synthesized by aldol condensation of benzaldehyde and
(c)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized using aldol condensation but not in a good in good yield. The starting material is
Explanation of Solution
The given compound is shown below.
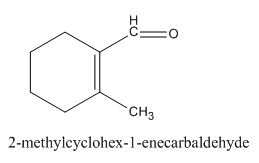
Figure 5
In the preparation of the above compound, intramolecular aldol condensation takes place. To form this compound enolate must be generated by aldehyde and it has to attack the carbonyl carbon of ketone which is not favorable condition. Favorable condition is that enolate should be generated on ketone which attacks the electrophilic carbon of the aldehyde. This is because the carbon of aldehyde is more acidic than the carbon of ketone. The structure of the starting material which is used to produce the given compound is shown below.
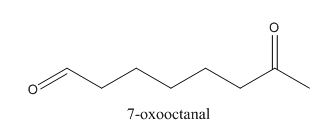
Figure 6
Therefore, the given compound cannot be synthesized by aldol condensation in a good yield.
This compound cannot be synthesized in a good yield by the aldol condensation.
(d)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation in good yield. The starting material is
Explanation of Solution
The given compound is shown below.
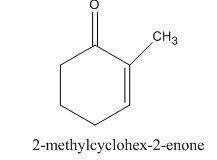
Figure 7
The given compound, is prepared by the intramolecular aldol condensation of
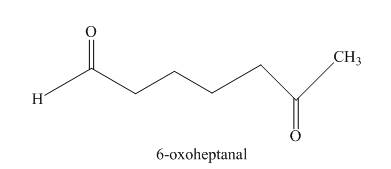
Figure 8
The given compound can be synthesized by intramolecular aldol condensation of
(e)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized from aldol condensation in a good yield. The starting materials are:
Explanation of Solution
The given compound is shown below.
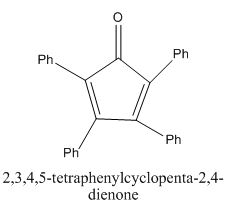
Figure 9
In the preparation of the given compound, the enolate will be generated on the
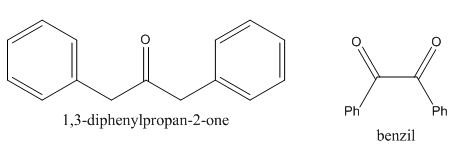
Figure 10
The given compound can be synthesized by aldol condensation of
(f)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation in a good yield. The starting materials are
Explanation of Solution
The given compound is shown below.
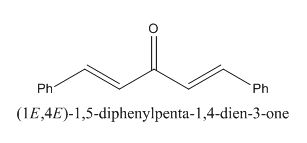
Figure 11
In the preparation of the compound, the enolate is generated on the
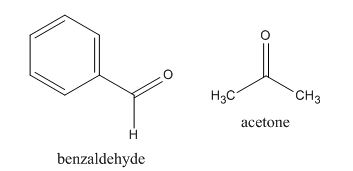
Figure 12
The given compound can be synthesized by aldol condensation of benzaldehyde and acetone.
(g)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation. The starting materials are trimethylacetone and acetaldehyde.
Explanation of Solution
The given compound is shown below.
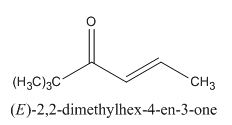
Figure 13
In the preparation of the given compound, the enolate will be generated on the
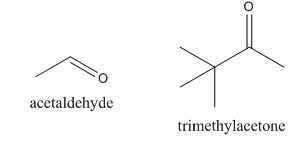
Figure 14
The given compound can be synthesized by aldol condensation by trimethylacetone and acetaldehyde in a good yield.
(h)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation in a good yield. The starting material is
Explanation of Solution
The given compound is shown below.
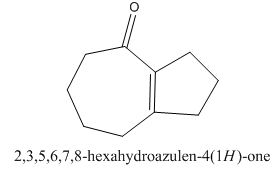
Figure 15
The enolate will be generated on the
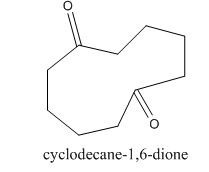
Figure 16
The given compound can be synthesized by aldol condensation of
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- When phenylacetaldehyde (C6H5CH2CHO) is dissolved in D2O with added DCl, the hydrogen atoms a to the carbonyl are gradually replaced by deuterium atoms. Write a mechanism for this process that involves enols as intermediates.arrow_forwardThe following compound may be synthesized through alkylation of an appropriate enamine with an alkyl bromide, followed by hydrolysis of the resulting immonium ion. Using this strategy, provide the necessary starting materials for the synthesis. Draw the necessary enamine below.arrow_forwardAcetone reacts with carbohydrates in the presence of an acid catalyst to form products that are commonly referred to as “isopropylidene” or “acetonide” derivatives. The carbohydrate d-ribono-(1,4)-lactone reacts with acetone in the presence of hydrochloric acid to give the acetonide shown here. Write a mechanism for this reaction.arrow_forward
- The ketone shown was prepared in a three-step sequence from ethyl trifluoroacetate. The first step in the sequence involved treating ethyl trifluoroacetate with ammonia to give compound A. Compound A was in turn converted to the desired ketone by way of compound B. Fill in the missing reagents in the sequence shown, and give the structures of compounds A and B.arrow_forward2,4-Dinitrophenylhydrazine is frequently used for making derivatives of ketones andaldehydes because the products (2,4-dinitrophenylhydrazones, called 2,4-DNP derivatives)are even more likely than the phenylhydrazones to be solids with sharp melting points.Propose a mechanism for the reaction of acetone with 2,4-dinitrophenylhydrazine in amildly acidic solution.arrow_forwardExplain how benzaldehyde and dimedone reacts with each other, and then with the aminotriazole to form compound 1a in the presence of an acid catalyst. Provide a detailed reaction mechanism and explanation.arrow_forward
- Methamphetamine is a long-lasting, potent stimulant sold as a street drug. The synthesis is quite simple and one step in the synthesis is shown below. Using your knowledge of the reactions of amines, provide all the reagents necessary to complete the reaction below. Provide the reagents for step 1 and step 2 ,arrow_forwardOrganic chemists work with tetraphenylporphyrins rather than with porphyrins because tetraphenylporphyrins are much more resistant to air oxidation. Tetraphenylporphyrin can be prepared by the reaction of benzaldehyde with pyrrole. Propose a mechanism for the formation of the ring system shown here:arrow_forwardProvide a mechanism for the following transformationarrow_forward
- A common illicit synthesis of methamphetamine involves an interesting variation of the Birch reduction. A solution of ephedrine in alcohol is added to liquid ammonia, followed by several pieces of lithium metal. The Birch reduction usually reduces the aromatic ring, but in this case it eliminates the hydroxy group of ephedrine to give methamphetamine. Propose a mechanism, similar to that for the Birch reduction, to explain this unusual course of the reaction.arrow_forwardAcid-catalyzed hydrolysis of the following epoxide gives a trans diol. Of the two possible trans diols, only one is formed. How do you account for this stereoselectivity?arrow_forwardFollowing is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
