
(a)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.56AP
The principal organic product obtained when
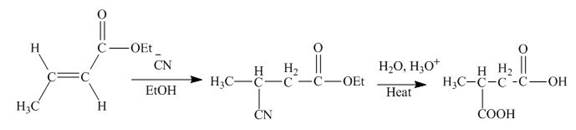
Explanation of Solution
The principal organic product obtained when

Figure 1
In this reaction, the addition of
The principal organic product expected when
(b)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.56AP
The principal organic product obtained when
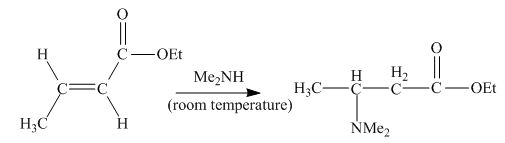
Explanation of Solution
The principal organic product obtained when

Figure 2
In this reaction, the addition of
The principal organic product obtained when
(c)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.56AP
The principal organic product obtained when
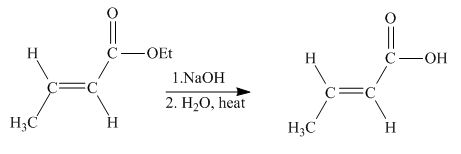
Explanation of Solution
The principal organic product obtained when
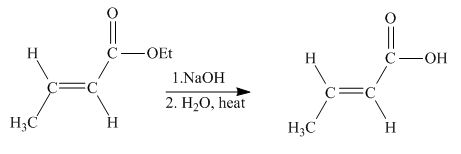
Figure 3
The
The principal organic product obtained when
(d)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.56AP
The principal organic product obtained when
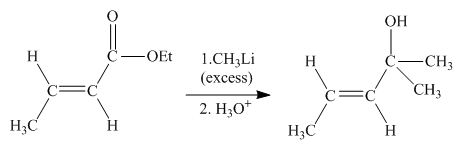
Explanation of Solution
The principal organic product obtained when

Figure 4
In this reaction,
The principal organic product obtained when
(e)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.56AP
The principal organic product obtained when
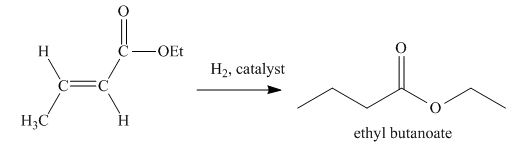
Explanation of Solution
The principal organic product obtained when

Figure 5
In this reaction, the addition of
The principal organic product obtained when
(f)
Interpretation:
The principal organic product expected when
Concept introduction:
Diels-Alder reaction is a cycloaddition reaction. The reaction is known as a
Answer to Problem 22.56AP
The principal organic product obtained when
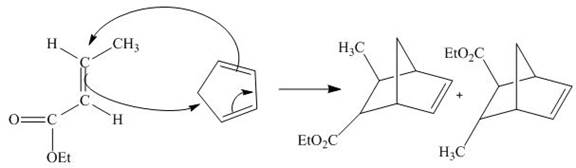
Explanation of Solution
The principal organic product obtained when

Figure 6
The reaction of
The principal organic product obtained when
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Amines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardTreatment of 1-aminoadamantane, C10H17N, with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. Propose a structural formula for compound A.arrow_forwardDraw a resonance structure of the acetonitrile anion, -: CH2CN, and account for the acidity of nitriles.arrow_forward
- Dihydropyran is synthesized by treating tetrahydrofurfuryl alcohol with an arenesulfonic acid, ArSO3H. Propose a mechanism for this conversion.arrow_forwardGive the expected organic product when phenylacetic acid, PhCH2COOH, is treated with reagent Q.)NaOH, H2Oarrow_forwardOne frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2. The reaction occurs in two steps: (l) protonation of diazomethane by the carboxylic acid to yield methyldiazonium ion, CH3N2+, plus a carboxylate ion; and (2) reaction of the carboxylate ion with CH3N2+. (a) Draw two resonance structures of diazomethane, and account for step 1. (b) What kind of reaction occurs in step 2?arrow_forward
- The following sequence of steps converts (R)-2-octanol to (S)-2-octanol. Propose structural formulas for intermediates A and B, specify the configuration of each, and account for the inversion of configuration in this sequence.arrow_forwardWhen cis-2-decalone is dissolved in ether containing a trace of HCl, an equilibrium is established with trans-2-decalone. The latter ketone predominates in the equilibrium mixture. Propose a mechanism for this isomerization and account for the fact that the trans isomer predominates at equilibrium.arrow_forwardCompound H (C8H6O3) gives a precipitate when treated with hydroxylamine in aqueous ethanol and a silver mirror when treated with Tollens solution. Following is its 1H-NMR spectrum. Deduce the structure of compound H.arrow_forward
- A step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forward2-bromo-2-methylbutane undergoes hydrolysis reaction with water, H2O toform compound W. Compound X and compound Y are produced when 2-bromo-2-methylbutane undergoes elimination reaction with alcoholic ofsodium hydroxide, NaOH. (i) Draw the structural formula of compounds W, X and Yarrow_forwardSeveral sulfonylureas, a class of compounds containing RSO2NHCONHR, are useful drugs as orally active replacements for injected insulin in patients with adult-onset diabetes. These drugs decrease blood glucose concentrations by stimulating b cells of the pancreas to release insulin and by increasing the sensitivity of insulin receptors in peripheral tissues to insulin stimulation. Tolbutamide is synthesized by the reaction of the sodium salt of p-toluenesulfonamide and ethyl N-butylcarbamate . Propose a mechanism for this step.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

