
(a)
Interpretation:
The enol structure of the compound
Concept introduction:
Answer to Problem 22.7P
The enol structures of the compound
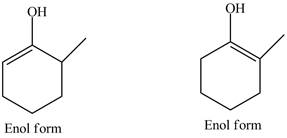
Explanation of Solution
The structure of
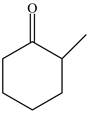
Figure 1
In the compound,
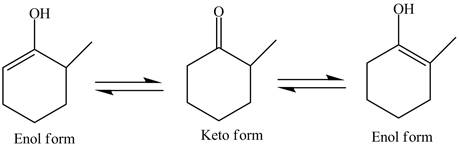
Figure 2
The compound
The enol structures of the compound
(b)
Interpretation:
The enol structure of the compound
Concept introduction:
Ketone also exists in two forms which are commonly known as keto-enol tautomerism. Tautomers are isomers which differ only in the position of the protons and electrons of the double bond of the electronegative atom in the compound. There is no change in the carbon skeleton of the compound. This phenomenon which involves simple proton transfer in an intramolecular fashion is known as tautomerism.
Answer to Problem 22.7P
The enol structure of the compound
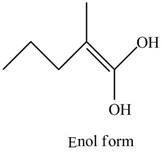
Explanation of Solution
The structure of given compound
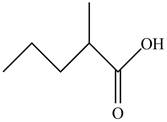
Figure 3
In the compound,
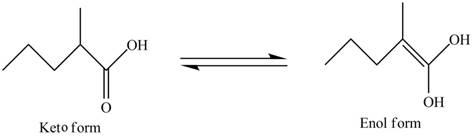
Figure 4
The enol structure of the compound
(c)
Interpretation:
The enol structure of the compound benzaldehyde is to be drawn. The explanation as to why the compound does not form enol structure.
Concept introduction:
The carbonyl compound contains a
Answer to Problem 22.7P
There is no
Explanation of Solution
The given compound benzaldehydeis shown below.
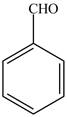
Figure 5
In the compoundbenzaldehydethere is no
In benzaldehyde, due to the absence of
(d)
Interpretation:
The enol structure of the compound
Concept introduction:
Ketone also exists in two forms which are commonly known as keto-enol tautomerism. Tautomers are isomers which differ only in the position of the protons and electrons of the double bond of the electronegative atom in the compound. There is no change in the carbon skeleton of the compound. This phenomenon which involves simple proton transfer in an intramolecular fashion is known as tautomerism.
Answer to Problem 22.7P
The enol structure of the compound
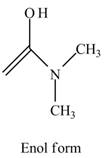
Explanation of Solution
The structure of the given compound
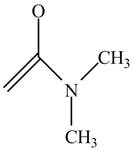
Figure 6
In the compound,
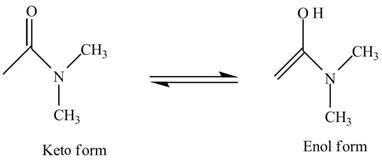
Figure 7
The enol structure of the compound
(e)
Interpretation:
The enol structure of the compound
Concept introduction:
Ketone also exists in two forms which are commonly known as keto-enol tautomerism. Tautomers are isomers which differ only in the position of the protons and electrons of the double bond of the electronegative atom in the compound. There is no change in the carbon skeleton of the compound. This phenomenon which involves simple proton transfer in an intramolecular fashion is known as tautomerism.
Answer to Problem 22.7P
There is no
Explanation of Solution
The given compound
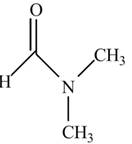
Figure 8
In the compound
In
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Rank the following compounds in order of increasing basicity: I. p-nitroaniline III. N-methylaniline II. p-aminobenzaldehyde IV. p-methylanilinearrow_forward(a) Give a plausible explanation for each one of the following :(i) There are two – NH2 groups in semicarbazide. However, only one such group is involved in the formation of semicarbazones.(ii) Cyclohexanone forms cyanohydrin in good yield but 2, 4, 6-trimethylcyclohexanone does not.(b) An organic compound with molecular formula C9H10O forms 2, 4, – DNP derivative, reduces Tollens’ reagent and undergoes Cannizzaro’s reaction. On vigorous oxidation it gives 1, 2-benzene-di- carboxylic acid. Identify the compound.arrow_forwardWhen acetophenone, hydroxelamine hydrochloride, and sodium hydroxide are reacted to synthesize acetophenone oxime, why is it necessary to add hydrochloric acid after the reaction to make it acidic?arrow_forward
- The ketone shown was prepared in a three-step sequence from ethyl trifluoroacetate. The first step in the sequence involved treating ethyl trifluoroacetate with ammonia to give compound A. Compound A was in turn converted to the desired ketone by way of compound B. Fill in the missing reagents in the sequence shown, and give the structures of compounds A and B.arrow_forwardExplain how benzaldehyde and dimedone reacts with each other, and then with the aminotriazole to form compound 1a in the presence of an acid catalyst. Provide a detailed reaction mechanism and explanation.arrow_forwardDescribe six way to prepare to 1-pentanamine and write two ways reaction routrs to obtain 1-pentene from the two way to prepare 1-pentanaminearrow_forward
- The analgesic acetaminophen is synthesized by treating 4-aminophenol with one equivalent of acetic anhydride. Complete the equation for the formation of acetaminophen. a. Acetaminophen: b. Additional Product pls add the name for both, thanks!arrow_forwarda. Would you expect hemiacetals to be stable in basic solutions? Explain your answer.b. Acetal formation must be catalyzed by an acid. Explain why it cannot be catalyzed by CH3O-.c. Can the rate of hydrate formation be increased by hydroxide ion as well as by acid? Explain.arrow_forwardArrange the members of each group in order of decreasing basicity: (a) Ammonia, aniline, methylamine (b) Acetanilide, aniline, N-methylaniline (c) 2,4-Dichloroaniline, 2,4-dimethylaniline, 2,4-dinitroaniline (d) 3,4-Dichloroaniline, 4-chloro-2-nitroaniline, 4-chloro-3-nitroaniline (e) Dimethylamine, diphenylamine, N-methylanilinearrow_forward
- Benzenesulfonyl chloride or p-toluenesulfonylchloride give N-substituted sulfonamides with primary and secondary amines. The derivative consisting of primary amines is insoluble in dilute NaOH.RightFalsearrow_forwardQuinapril (trade name Accupril) is used to treat high blood pressure andcongestive heart failure. One step in the synthesis of quinapril involvesreaction of the racemic alkyl bromide A with a single enantiomer of theamino ester B. Given the structure of quinapril, which one of these two products isneeded to synthesize the drug?arrow_forwardWhat reaction sequence will form dibenylamine from benzoic acid in high yieldarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

