
Concept explainers
(a)
Interpretation:
The closest
Concept introduction:
A unit cell of the crystal is the three-dimensional arrangement of the atoms present in the crystal. The unit cell is the smallest and simplest unit of the crystal which on repetition forms an entire crystal. Unit cell can be a cubic unit cell or hexagonal unit cell. The classification of a unit cell depends on the lattice site occupied by the atoms.
Answer to Problem 22.35E
The closest
Explanation of Solution
The structure of a face-centered cubic lattice is shown below.
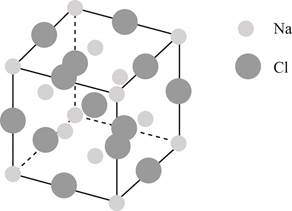
Figure 1
The plane of face-centered cubic lattice that has
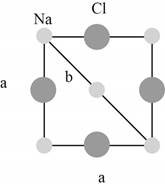
Figure 2
The closest
The lattice parameter
The Pythagoras theorem is shown below.
Where,
•
•
•
The hypotenuse of the triangle shown in Figure (2) is
The base and perpendicular of the triangle shown in Figure (2) are
Substitute the value of hypotenuse, base, and perpendicular in the equation (1).
Substitute the value of
Therefore, the closest
The closest
(b)
Interpretation:
The closest
Concept introduction:
A unit cell of the crystal is the three-dimensional arrangement of the atoms present in the crystal. The unit cell is the smallest and simplest unit of the crystal which on repetition forms an entire crystal. Unit cell can be a cubic unit cell or hexagonal unit cell. The classification of a unit cell depends on the lattice site occupied by the atoms.
Answer to Problem 22.35E
The closest
Explanation of Solution
The structure of a face-centered cubic lattice is shown below.
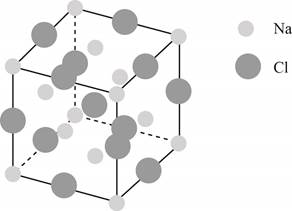
Figure 1
The plane of face-centered cubic lattice that has
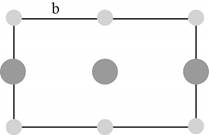
Figure 3
The lattice parameter
The relation between the length of the edge of a cube
The diagonal of the triangle shown in Figure (3) is
Substitute the value of
Therefore, the closest
The closest
(c)
Interpretation:
The closest
Concept introduction:
A unit cell of the crystal is the three-dimensional arrangement of the atoms present in the crystal. The unit cell is the smallest and simplest unit of the crystal which on repetition forms an entire crystal. Unit cell can be a cubic unit cell or hexagonal unit cell. The classification of a unit cell depends on the lattice site occupied by the atoms.
Answer to Problem 22.35E
The closest
Explanation of Solution
The structure of a face-centered cubic lattice is shown below.
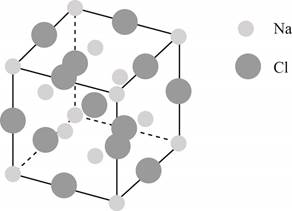
Figure 1
The plane of face-centered cubic lattice that has
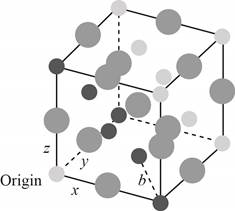
Figure 4
The side of the trigonal plane is same as the diagonal of the of the cube.
The lattice parameter
The relation between the length of the edge of a cube
The diagonal of the triangle shown in Figure (4) is
Substitute the value of
Therefore, the closest
The closest
Want to see more full solutions like this?
Chapter 22 Solutions
Physical Chemistry
- Nanotechnology, or technology utilizing 1100 nm sized particles, has rapidly expanded in the past few decades, with potential applications ranging across far-reaching fields such as electronics, medicine, biomaterials, and consumer products, to name a few. One of the primary advantages of nanoparticles is the presence of large surface/mass ratios, resulting in enhanced surface activities compared to bulk materials. a Use the density of silver (10.49 g/cm3) to determine the number of Ag atoms in a spherical 20.-nm silver particle. b In the crystalline metallic environment, the measured radii of silver atoms has been measured to be 144 pm. Use this to calculate the atomic packing fraction of a 20.-nm silver particle. In other words, calculate the ratio of the volume taken up by Ag atoms to the volume of the entire nanoparticle. c Based on the result of part (b), silver conforms to which type of cubic crystal lattice? A simple cubic B body-centered cubic C face-centered cubic d A cubic Ag ingot having a mass of 5.0-g is processed to form a batch of 20.-nm Ag nanoparticles. Calculate the ratio of the surface area provided by the batch of nanoparticles to the surface area of the initial cube of Ag.arrow_forwardExpress the relationship between atomic radius (r) and the edge length (a) in the bcc unit cell.arrow_forwardExpress the relationship between atomic radius(r) and the edge length (a) of the fee unit cell.arrow_forward
- 1. What is the edge length of a simple cubic unit cell made up of atoms having a radius of 158 pm?2. State the number of atoms on a plane having the miller indices of 110 in a BCC unit cell.arrow_forwardwhat is the density of tantalum in a unit cell g/cm^3 with a z number of 2 and a pm of 331.arrow_forwardCalculate the volume of the unit cell of Zn crystal. a andc of Zn are 266.5 pm and 494.7 pm respectively.arrow_forward
- What is amount of space (in nm^3) occupied by atoms in the face-centered cubic whose side is 2 nm?arrow_forwardG. Bakale et al. (J. Phys. Chem., 12477 (1996)) measured the mobility of singly charged C60− ions in a variety of nonpolar solvents. In cyclohexane at 22 °C (viscosity is 0.93 × 10−3 kg m−1 s−1), the mobility is 1.1 × 10−4 cm2 V−1 s−1. Estimate the effective radius of the C60− ion. Suggest a reason why there is a substantial difference between this number and the van der Waals radius of neutral C60.arrow_forwardThe orthorhombic unit cell of NiSO4 has the dimensions a= 634 pm, b = 784 pm, and c = 516 pm, and the density of the solid is estimated as 3.9 g cm-3. Determine the number of formula units per unit cell and calculate a moreprecise value of the density.arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





