
Concept explainers
Draw the product formed when pentanoyl chloride
a.
b.
(a)
Interpretation:
The product formed by the treatment of pentanoyl chloride
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilic acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.45P
The product formed by the treatment of pentanoyl chloride
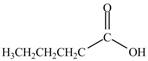
Explanation of Solution
Acyl chloride on reaction with
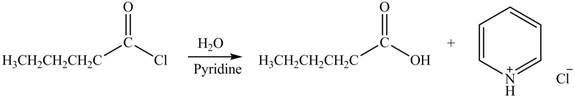
Figure 1
The product formed by the treatment of pentanoyl chloride
(b)
Interpretation:
The product formed by the treatment of pentanoyl chloride
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilic acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.45P
The product formed by the treatment of pentanoyl chloride
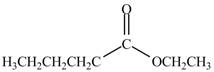
Explanation of Solution
The acyl chloride on reaction with alcohol converts into ester. The oxygen atom of alcoholic
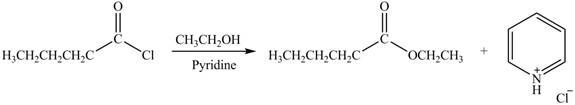
Figure 2
The product formed by the treatment of pentanoyl chloride
(c)
Interpretation:
The product formed by the treatment of pentanoyl chloride
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilic acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.45P
The product formed by the treatment of pentanoyl chloride
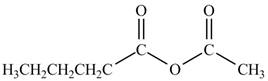
Explanation of Solution
The acyl chloride on reaction with acetate ion converts into acid anhydride. The oxygen atom of acetate ion contains negative charge. It undergoes nucleophilic attack on electron deficient carbonyl carbon atom and replaces chlorine atom. The reaction between pentanoyl chloride and
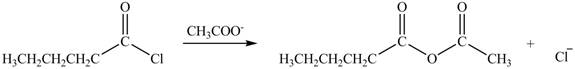
Figure 3
The product formed by the treatment of pentanoyl chloride
(d)
Interpretation:
The product formed by the treatment of pentanoyl chloride
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilic acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.45P
The product formed by the treatment of pentanoyl chloride
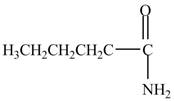
Explanation of Solution
The reaction of acyl chloride with ammonia
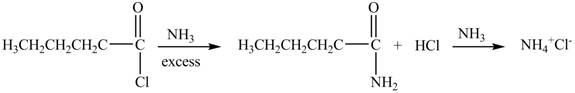
Figure 4
The product formed by the treatment of pentanoyl chloride
(e)
Interpretation:
The product formed by the treatment of pentanoyl chloride
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilic acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.45P
The product formed by the treatment of pentanoyl chloride
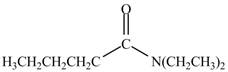
Explanation of Solution
The reaction of acyl chloride with secondary amine leads to the formation of amide. The nitrogen atom of
![]()
Figure 5
The product formed by the treatment of pentanoyl chloride
(f)
Interpretation:
The product formed by the treatment of pentanoyl chloride
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilic acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.45P
The product formed by the treatment of pentanoyl chloride
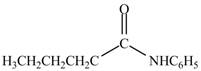
Explanation of Solution
The reaction of acyl chloride with primary amine leads to the formation of amide. The nitrogen atom of

Figure 6
The product formed by the treatment of pentanoyl chloride
Want to see more full solutions like this?
Chapter 22 Solutions
ALEKS 360 CHEMISTRY ACCESS
- Draw the products formed when each alkene is treated with HCl.arrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent. a.SOCl2, pyridine b. TsCl, pyridine c.H2SO4 d.HBr e.PBr3, then NaCN f.POCl3, pyridinearrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent. a. SOCl2, pyridine b. TsCl, pyridine c. H2SO4 d. HBr e. PBr3, then NaCN f. POCl3, pyridinearrow_forward
- Draw the products formed when D-altrose is treated with each reagent. a. (CH3)2CHOH, HCl b. NaBH4, CH3OH c. Br2, H2O d. HNO3, H2O e. [1] NH2OH; [2] (CH3CO)2O, NaOCOCH3; [3] NaOCH3 f. [1] NaCN, HCl; [2] H2, Pd-BaSO4; [3] H3O+ g. CH3I, Ag2O h. C6H5CH2NH2, mild H+arrow_forwardDraw the missing starting material. Reagent 1 is benzene and AlCl3. Reagent B is Zn(Hg) and HCl.arrow_forward1. What functional group is produced when an aldehyde reacts with H2/Pt? A.secondary alcohol B. carboxylic acid C.hemiacetal D. primary alcohol E.alkane F.tertiary alcohol G. alkene 2. What reaction occurs when an aldehyde reacts with H2/Pt to form a primary alcohol? A. Hydration B. Hydration C. Dehydration D. Oxidation E. Reduction( hydrogentation) 3. What reaction occurs when an Ester react with H+/H2O to from a carboxylic acid and alcohol? A. Dehydration B. Reduction ( Hydrogenation) C.Hydrolysis D. Hydration E.oxidationarrow_forward
- Draw the product formed when (CH3)2CHOH is treated with following reagent. H2SO4arrow_forwardDraw the products formed when (CH3)2C=CH2 is treated with each reagent.a. HBrb. H2OH2SO4c. CH3CH2OH, H2SO4d. Cl2e. Br2, H2Of. NBS (aqueous DMSO)g. [1]BH3;[2]H2O2, HO-arrow_forwardDraw the products formed when (CH3)2C=CH2 is treated with following reagent. [1] BH3; [2] H2O2, HO−arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





