
Concept explainers
(a)
Interpretation: The aldol product that is formed from the given pair of starting materials by using
Concept Introduction: Aldol reaction is the condensation reaction of the
Answer to Problem 29P
The aldol product that is formed from the given pair of starting materials by using
Explanation of Solution
The given ball and stick model of the pair of compounds is shown as,
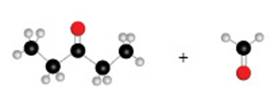
Figure 1
According to the given ball and stick model, the given pair of compounds is identified as,
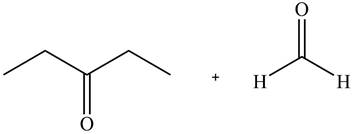
Figure 2
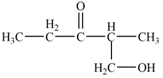
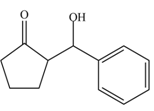
The aldol product that is formed from the given pair of starting materials by using
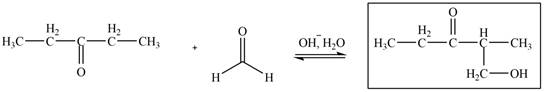
Figure 3
In this step, the base,
The aldol product that is formed from the given pair of starting materials by using
(b)
Interpretation: The aldol product that is formed from the given pair of starting materials by using
Concept Introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 29P
The aldol product that is formed from the given pair of starting materials by using
Explanation of Solution
The given ball and stick model of the pair of compounds is shown as,
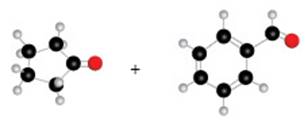
Figure 4
According to the given ball and stick model, the given pair of compounds is identified as,
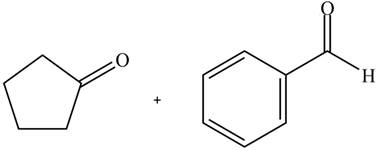
Figure 5
The aldol product that is formed from the given pair of starting materials by using
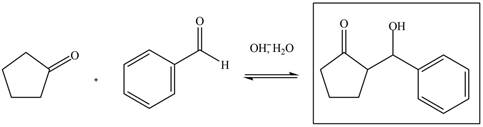
Figure 6
In this step, the base,
The aldol product that is formed from the given pair of starting materials by using
Want to see more full solutions like this?
Chapter 22 Solutions
Package: Loose Leaf For Organic Chemistry With Connect Access Card (1 Semester)
- Which statement describes why aldol reactions with ketones are low yielding? A.Ketones do not have acidic protons. B.Ketones are too electron poor at the carbonyl carbon. C.Ketones can attack other ketones well but perform poorly in self-condensations. D.The product of a ketone addition to a ketone has considerable steric strain.arrow_forwardIn an aldol condensation reaction, acetone and benzaldehyde are mixed in the presence of sodium hydroxide to produce dibenzalacetone. Does it matter whether the acetone is added first to the aq. NaOH solution then the benzaldehyde, or if the benzaldehyde is added first to the aq. NaOH solution, then the acetone? Explainarrow_forwardDraw possible products of aldol reaction. Including dehydrated aldol products.arrow_forward
- What is the best reaction to form a beta-ketone? 1. Aldol-Claisen Condensation 2. Aldol-Claisen Addition 3. Aldol Condensation 4. Claisen Condensationarrow_forwardDraw out the machanism for the aldol condensation of (2E,6E)-2,6-bis(4-ethylbenzylidene)cyclohexanone. (the reaction between cyclohexanone and 4-methoxybenzaldehyde)arrow_forwardDraw the product of the base-catalyzed aldol reaction of compound. Q.) Cyclopentanonearrow_forward
- What are Directed Aldol Reactions ?arrow_forwardWhat is the first step in a base catalyzed aldol reaction? A. Protonation of Carbonyl group B. Elimination of Carbonyl with an alkene C. Formation of an Enolate D. Elimination of hydroxide E. Addition of nucleophile to R2C=Oarrow_forwardThe aldol double condensation reaction is highly selective for formation of a single double condensation product. Explain why only one aldol condensation product is formed despite two carbonyl compounds being present for the reaction.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
