
ORGANIC CHEMISTRY (LL)-W/STUDY GUIDE
8th Edition
ISBN: 9780134653631
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 40P
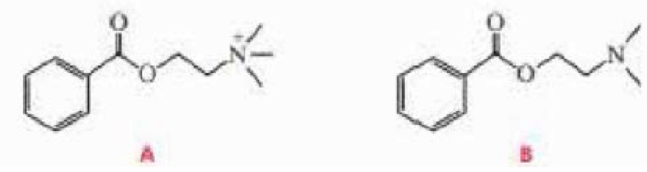
At pH = 12, the rate of hydrolysis of ester A is greater than the rate of hydrolysis of ester B. At pH = 8, the relative rates reverse (that is, ester B hydrolyzes faster than ester A). Explain these observations.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Put these three common types of carbonyl compound in order of decreasing reactivity
ester amide acid chloride
2. For the least reactive, show the interconversion to its other resonance form: How does this electron delocalisation make it stable?
3. For the most reactive, draw the mechanism of its undergoing hydrolysis (reaction with H2O):
Why makes this type of carbonyl so reactive to nucleophiles?
Order the following in increasing acid strength and explain your reasoning.
a. Benzoic Acid, b. 4-nitrobenzoic acid, c. 4-methylbenzoic acid, d. 4-methoxybenzoic acid
Maleic Acid + Bromine à racemic-2,3-Dibromosuccinic Acid
Explain the mechanism and reaction
Chapter 22 Solutions
ORGANIC CHEMISTRY (LL)-W/STUDY GUIDE
Ch. 22.2 - Compare each of the mechanisms listed here with...Ch. 22.2 - Prob. 3PCh. 22.2 - Prob. 4PCh. 22.3 - a. Draw the mechanism for the following reaction...Ch. 22.5 - Prob. 7PCh. 22.5 - Propose a mechanism for the Co2+ catalyzed...Ch. 22.6 - Prob. 9PCh. 22.7 - Prob. 10PCh. 22.7 - Prob. 12PCh. 22.7 - Prob. 13P
Ch. 22.9 - Which of the following amino acid side chains can...Ch. 22.9 - Which of the following C-terminal peptide bonds is...Ch. 22.9 - Carboxypeptidase A has esterase activity as well...Ch. 22.10 - Arginine and lysine side chains fit into trypsins...Ch. 22.10 - Explain why serine proteases do not catalyze...Ch. 22.11 - If H2 18O is used in the hydrolysis reaction...Ch. 22.11 - Draw the pH-activity profile for an enzyme that...Ch. 22.12 - The pHactivity profile for glucose-6-phosphate...Ch. 22.12 - Prob. 23PCh. 22.13 - Draw the mechanism for the hydroxide ion-catalyzed...Ch. 22.13 - What advantage does the enzyme gain by forming an...Ch. 22.13 - Prob. 26PCh. 22.13 - Prob. 27PCh. 22.13 - Aldolase shows no activity if it is incubated with...Ch. 22 - Which of the following parameters would be...Ch. 22 - Prob. 29PCh. 22 - Prob. 30PCh. 22 - Prob. 31PCh. 22 - Indicate the type of catalysis that is occurring...Ch. 22 - The deuterium kinetic isotope effect (KH2O/KD2O)...Ch. 22 - Prob. 34PCh. 22 - Co2+ catalyzes the hydrolysis of the lactam shown...Ch. 22 - there are two kinds of aldolases. Class I...Ch. 22 - Prob. 37PCh. 22 - The hydrolysis of the ester shown here is...Ch. 22 - Prob. 39PCh. 22 - At pH = 12, the rate of hydrolysis of ester A is...Ch. 22 - 2-Acetoxycyclohexyl tosylate reacts with acetate...Ch. 22 - Proof that an imine was formed between aldolase...Ch. 22 - Prob. 43PCh. 22 - a. Explain why the alkyl halide shown here reacts...Ch. 22 - Triosephosphate isomerase (TIM) catalyzes the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 10. What is the order of increase in acidity for the following compounds?arrow_forwardWhat would be the best base to use to conduct a Claisen Condensation reaction with the following ester?arrow_forwardPropose a mechanism for the acid-catalyzed hydration of propene. Remember that a mechanism must include curved arrows to show movement of electrons, as well as all intermediates.arrow_forward
- Arrange the following in the order of decreasing reactivity towards a nucleophile (number them 1-5) Acid amiide Acid halide Ester Carboxylic acid Acid anhydride Rationalize the order of reactivity and explain whyarrow_forwardNot sure how to approach this problem.arrow_forwardExplain this statement: Although 2-methoxyacetic acid (CH3OCH2COOH)is a stronger acid than acetic acid (CH3COOH), p-methoxybenzoic acid(CH3OC6H4COOH) is a weaker acid than benzoic acid (C6H5COOH).arrow_forward
- a) Put these three common types of carbonyl compound in order of decreasing reactivity ester amide acid chloride b) For the least reactive, show the interconversion to its other resonance form: How does this electron delocalisation make it stable? c) For the most reactive, draw the mechanism of its undergoing hydrolysis (reaction with H2O): Why makes this type of carbonyl so reactive to nucleophiles?arrow_forwarda. Compound X is benzene, Y is acetic anhydride acid. Complete the following scheme and determine Z! b. Determine which reagents except acetic acid anhydrides can replace Y!arrow_forwardWhich or which of the statements given below is correct. I) Maleic anhydride is a carboxylic acid derivative and its reaction with water is a reduction reaction. II) Fumaric acid and maleic acid are stereoisomers of each other III) Since fumaric acid has a more stable structure than maleic acid, its boiling point is higher. A. Solo I B. I and III C. II and III D. I, II, III E. Solo IIIarrow_forward
- Show the product formed as a result of the reaction between propanoic acid and benzylalcohol in an acidic environment by writing the reaction mechanism together.arrow_forwardList the appropriate reagents that can be used in places with question marks in the following series of reactions.arrow_forwardI need to show a mechanism for trans cinnamic acid reacting with dibromide to get (2S, 3R)-dibromo-3-phenylpropanoic acid If you could explain what is happening during the mechanism as well as the mechanism itself, I would greatly appreciate itarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License