
Concept explainers
(a)
Interpretation:
Condensed structural formula for the given set of compounds has to be written.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an
Root word represents the longest continuous carbon skeleton of the organic molecule.
The structure of a molecule can be drawn by analyzing the presence of prefix, suffix and root word in the given IUPAC name.
Condensed structural formula is representation of the organic compound. In this the lengthy carbon chain is shown only with the carbon atoms (along with the hydrogen) without any bonds. The additional branches are shown with explicit bonds.
To Write: The condensed structural formula for the given compounds.
(a)
Answer to Problem 23.39QP
The condensed structural formula for 2,5-dimethyloctane is,
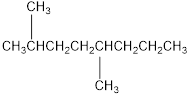
Explanation of Solution
From the given name of the hydrocarbon, the parent carbon is identified as octane. Octane has eight carbon chain. In the given name it is given as two methyl groups are substituted in second and fifth carbon atom resulting in the skeleton of the hydrocarbon.
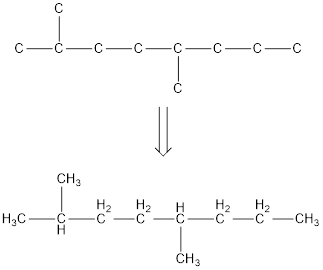
Remove the single bonds in the lengthy chain to obtain the condensed structural formula.
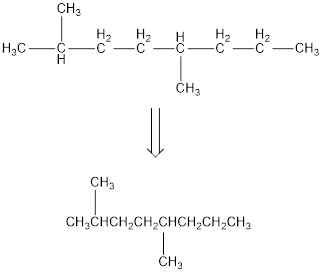
(b)
Interpretation:
Condensed structural formula for the given set of compounds has to be written.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc.
Root word represents the longest continuous carbon skeleton of the organic molecule.
The structure of a molecule can be drawn by analyzing the presence of prefix, suffix and root word in the given IUPAC name.
Condensed structural formula is representation of the organic compound. In this the lengthy carbon chain is shown only with the carbon atoms (along with the hydrogen) without any bonds. The additional branches are shown with explicit bonds.
To Write: The condensed structural formula for the given compounds.
(b)
Answer to Problem 23.39QP
The condensed structural formula for 4-ethyl-2-methylheptane is,
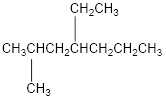
Explanation of Solution
From the given name of the hydrocarbon, the parent carbon is identified as heptane. Heptane has seven carbon chain. In the given name, it is given as a methyl group and ethyl group are substituted in fourth and second carbon atom respectively, resulting in the skeleton of the hydrocarbon.
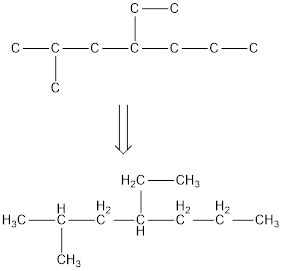
Remove the single bonds in the lengthy chain to obtain the condensed structural formula.
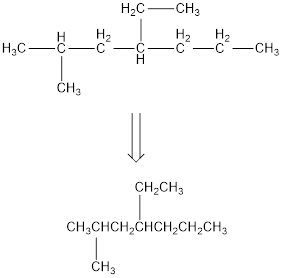
(c)
Interpretation:
Condensed structural formula for the given set of compounds has to be written.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc.
Root word represents the longest continuous carbon skeleton of the organic molecule.
The structure of a molecule can be drawn by analyzing the presence of prefix, suffix and root word in the given IUPAC name.
Condensed structural formula is representation of the organic compound. In this the lengthy carbon chain is shown only with the carbon atoms (along with the hydrogen) without any bonds. The additional branches are shown with explicit bonds.
To Write: The condensed structural formula for the given compounds.
(c)
Answer to Problem 23.39QP
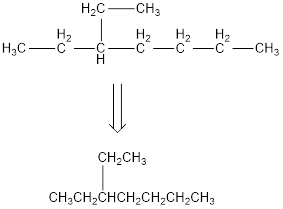
The condensed structural formula for 3-ethylheptane is,
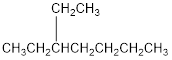
Explanation of Solution
From the given name of the hydrocarbon, the parent carbon is identified as heptane. Heptane has seven carbon chain. In the given name it is given as an ethyl group is substituted in third carbon atom resulting in the skeleton of the hydrocarbon.
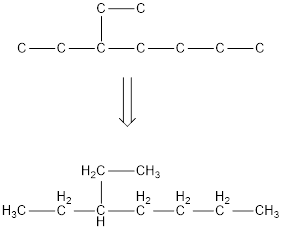
Remove the single bonds in the lengthy chain to obtain the condensed structural formula.
(d)
Interpretation:
Condensed structural formula for the given set of compounds has to be written.
Concept Introduction:
Any organic molecule can be named by using certain rules given by IUPAC (International Union for Pure and applied chemistry). IUPAC name consists of three parts in major namely Prefix suffix and root word.
Prefix represents the substituent present in the molecule and its position in the root name.
Suffix denotes the presence of functional group if any in the molecule. It can be an alkene, alkyne, alcohol, carboxylic acid, alcohol etc.
Root word represents the longest continuous carbon skeleton of the organic molecule.
The structure of a molecule can be drawn by analyzing the presence of prefix, suffix and root word in the given IUPAC name.
Condensed structural formula is representation of the organic compound. In this the lengthy carbon chain is shown only with the carbon atoms (along with the hydrogen) without any bonds. The additional branches are shown with explicit bonds.
To Write: The condensed structural formula for the given compounds.
(d)
Answer to Problem 23.39QP
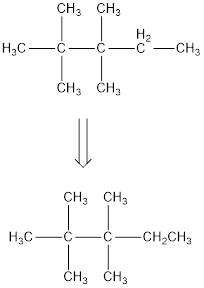
The condensed structural formula for 2,2,3,3-tetramethylpentane is,
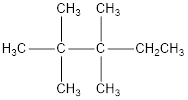
Explanation of Solution
From the given name of the hydrocarbon, the parent carbon is identified as pentane. Pentane has five carbon chain. In the given name it is given as two methyl groups are substituted in second carbon and two methyl groups are substituted in third carbon atom resulting in the skeleton of the hydrocarbon.
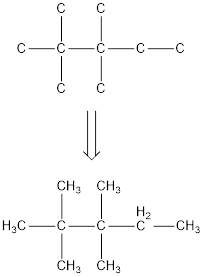
Remove the single bonds in the lengthy chain to obtain the condensed structural formula.
Want to see more full solutions like this?
Chapter 23 Solutions
Lab Manual Experiments in General Chemistry
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning  Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





