
Concept explainers
(a)
Interpretation:
The product obtained in the reaction of
Concept introduction:
Answer to Problem 23.44AP
The product obtained in the reaction of
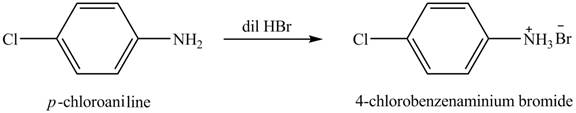
Explanation of Solution
When
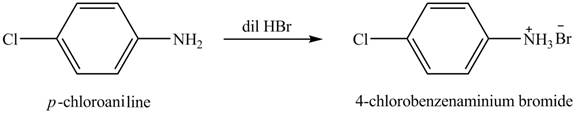
Figure 1
The product obtained in the reaction of
(b)
Interpretation:
The product obtained in the reaction of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. When alcohol reacts with hydrogen halide it forms
Answer to Problem 23.44AP
The product obtained in the reaction of
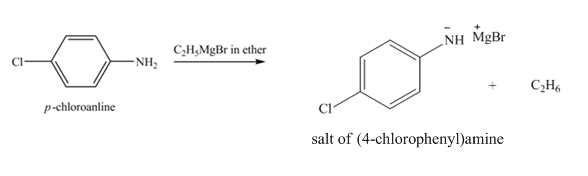
Explanation of Solution
The reaction of

Figure 2
The product obtained in the reaction of
(c)
Interpretation:
The product obtained in the reaction of
Concept introduction:
The formation of diazonium salt from
Answer to Problem 23.44AP
The product obtained in the reaction of
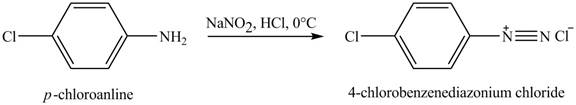
Explanation of Solution
When

Figure 3
The product obtained in the reaction of
(d)
Interpretation:
The product obtained in the reaction of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.44AP
The product
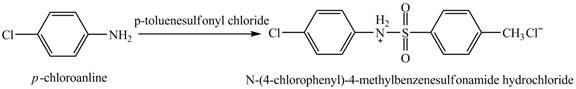
Explanation of Solution
When

Figure 4
The product
(e)
Interpretation:
The product obtained in the reaction of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. The formation of diazonium salt from aromatic amines takes place using sodium nitrite and hydrochloric acid at low temperatures. Aryl diazonium salts undergo a variety of specific substitution reactions in which the incoming Z group replaces
Answer to Problem 23.44AP
The product
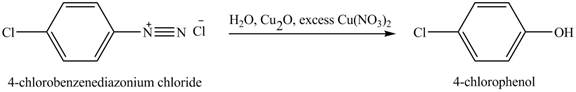
Explanation of Solution
When
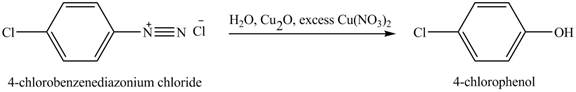
Figure 5
The product obtained in the reaction of
(f)
Interpretation:
The product obtained in the reaction of
Concept introduction:
The formation of diazonium salt from aromatic amines takes place using sodium nitrite and hydrochloric acid at low temperatures. Aryl diazonium salts undergo a variety of specific substitution reactions in which the incoming Z group replaces
Answer to Problem 23.44AP
The product
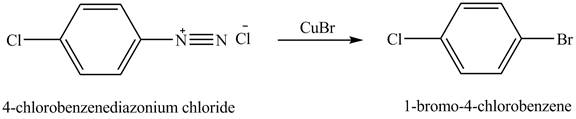
Explanation of Solution
When
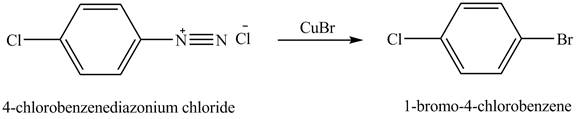
Figure 6
The product
(g)
Interpretation:
The product obtained in the reaction of
Concept introduction:
The formation of diazonium salt from aromatic amines takes place using sodium nitrite and hydrochloric acid at low temperatures. Aryl diazonium salts undergo a variety of specific substitution reactions in which the incoming Z group replaces
Answer to Problem 23.44AP
The product chlorobenzene is obtained in the reaction of the product of part (c) and
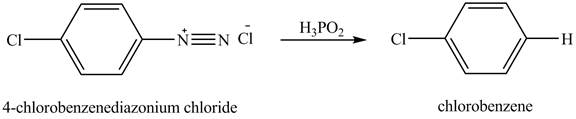
Explanation of Solution
The reduction reaction of
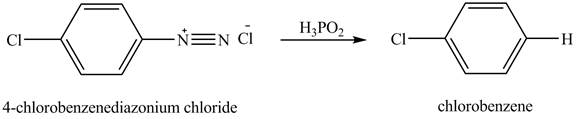
Figure 7
The product chlorobenzene is obtained in the reaction of the product of part (c) and
(h)
Interpretation:
The product obtained in the reaction of
Concept introduction:
The formation of diazonium salt from aromatic amines takes place using sodium nitrite and hydrochloric acid at low temperatures. Aryl diazonium salts undergo a variety of specific substitution reactions in which the incoming Z group replaces
Answer to Problem 23.44AP
The product,
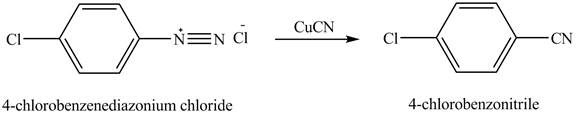
Explanation of Solution
When

Figure 8
The product,
(i)
Interpretation:
The product obtained in the reaction of
Concept introduction:
The formation of diazonium salt from aromatic amines takes place using sodium nitrite and hydrochloric acid at low temperatures. Aryl diazonium salts undergo a variety of specific substitution reactions in which the incoming Z group replaces N2 (a very good leaving group) to form corresponding products.
Answer to Problem 23.44AP
The product
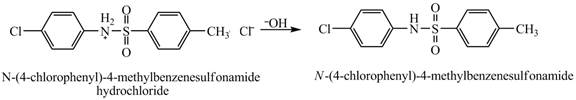
Explanation of Solution
When

Figure 9
The product
Want to see more full solutions like this?
Chapter 23 Solutions
EBK ORGANIC CHEMISTRY STUDY GUIDE AND S
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





