To determine whether high-spin and low-spin electron configuration in an octahedral field is possible, we need to determine the number of d electrons in each ion. Then we need to distribute them among the sets of d-orbitals split by an octahedral field to see if it is possible to have ions to have different spin states. The ground state electronic configurations of the four elements are Ar3d64s2 for Fe, Ar3d74s2 for Co, Ar3d54s2 for Mn, and Ar3d54s1 for Cr. When they form cations, the atoms of these transition metals first lose their 4s electrons and then their 3d electrons. Therefore, the numbers of d-electrons in the ions are 6 in Fe2+, 6 in Co3+, 5 in Mn2+, and 3 in Cr3+. It is likely that the ion with the fewest 3d-electrons (Cr3+) and the one with the most (Fe2+and Co3+) will have either a high-spin or a low-spin state.
Electronic configurations of cations and d-electrons are
| Ion |
Electronic configuration |
d-electrons |
| Fe2+ |
Ar 3d6 |
6 |
| Co3+ |
Ar 3d6 |
6 |
| Mn2+ |
Ar 3d5 |
5 |
| Cr3+ |
Ar 3d3 |
3 |
a) Fe2+: Six d- electrons can be arranged in octahedral field to form high spin and low spin complexes as follows:
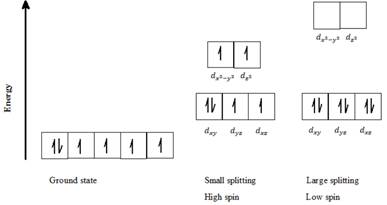
For a high spin complex small ∆o, following Hund’s rule, putting five electrons into the five d-orbitals of lower and higher energies respectively, and keeping them as unpaired as possible gives the orbital distribution of electrons, as seen in the above diagram. The sixth electron is paired up with the first electron in dxy orbital.
For a low spin complex with large ∆o, all six electrons are paired up in the lower set of d orbitals.
Thus, Fe2+ can exist in high spin as well as in low spin electron configuration in an octahedral field.
b) Co3+: Six d- electrons can be arranged in octahedral field to form high spin and low spin complexes as follows:
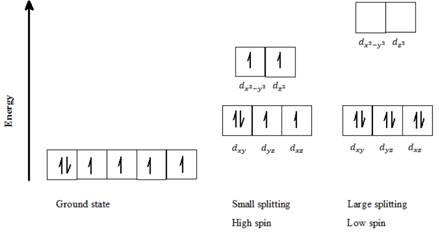
For a high spin complex small ∆o, following Hund’s rule, putting five electrons into the five d-orbitals of lower and higher energies respectively, and keeping them as unpaired as possible gives the orbital distribution of electrons as seen in the above diagram. The sixth electron is paired up with the first electron in dxy orbital.
For a low spin complex with large ∆o, all six electrons are paired up in the lower set of d orbitals.
Thus, Co3+ can exist in high spin as well as in low spin electron configuration in an octahedral field.
c) Mn2+: Five d- electrons can be arranged in octahedral field to form high spin and low spin complexes as follows:
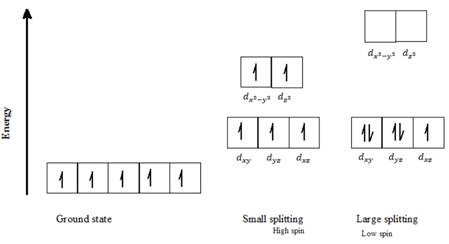
For a high spin complex small ∆o, following Hund’s rule, putting five electrons into the five d-orbitals of lower and higher energies respectively, and keeping them as unpaired as possible gives the orbital distribution of electrons, as seen in the above diagram.
For a low spin complex with large ∆o, following Hund’s rule, putting five electrons into the d-orbitals of lower energy, and keeping them as unpaired as possible gives the orbital distribution of electrons as seen in the above diagram. The fourth and fifth electron is paired up with the first two electrons, as seen in the above diagram for a low spin state.
Thus, Mn2+ can exist in high spin as well as in low spin electron configuration in an octahedral field.
d) Cr3+: Three d- electrons can be arranged in octahedral field to form high spin and low spin complexes as follows:
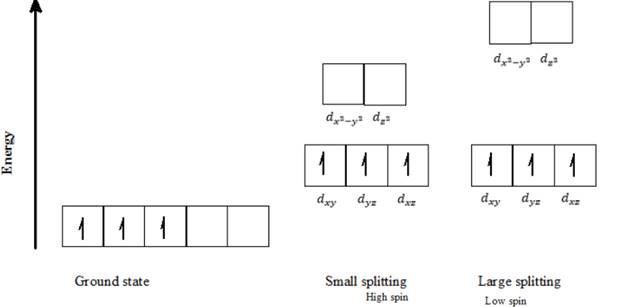
Three electrons in Cr3+ ion can be arranged in only one way. Putting three electrons into the lowest 3d orbitals available and keeping them as unpaired as possible gives this orbital distribution of electrons, as seen in the above diagram.
The metal ions with three or fewer d electrons have only one spin state, in which all the electrons are unpaired and in the lower energy set of orbitals. The Cr3+ has 3 d electrons, and thus it can have only one spin electron configuration in which all the electrons are unpaired, irrespective of the magnitude of crystal filed splitting (∆o).
Thus, Cr3+ can exist only in a high spin electron configuration in an octahedral field.
Conclusion:
Metal ions with 4, 5, 6, or 7 d electrons can exist in a high-spin and low-spin state depending on the magnitude of crystal field, splitting while those with 3 or fewer having only high-spin state, irrespective of the magnitude of crystal field splitting.





 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY




