
(a)
Interpretation:
The pH at which the zwitter ion [Z] and the cationic form [C+] of the amino acid alanine become equal needs to be calculated.
Concept introduction:
- Amino acids are organic compounds composed of C, H, N and O. The two main
functional groups include the amino −NH2 and carboxyl −COOH group in addition to a side chain each with a characteristic pKa value. - Isoelectric point (pI) is the pH at which the net charge on the amino acid is zero. For amino acid with one −COOH and one −NH2, the pI is given as:
Answer to Problem 30QAP
pH = 2.29
Explanation of Solution
The given amino acid is alanine which has the following structure:
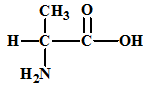
The structures of the respective cationic form (C+), zwitter ion (Z) and the anionic form (A-) are:
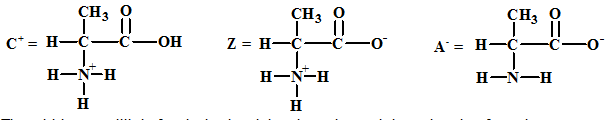
The acid-base equilibria for alanine involving the cation and the zwitter ion forms is:
(b)
Interpretation:
The pH at which the zwitter ion [Z] and the anionic form [A-] of the amino acid alanine become equal needs to be calculated
Concept introduction:
- Amino acids are organic compounds composed of C, H, N and O. The two main functional groups include the amino −NH2 and carboxyl −COOH group in addition to a side chain each with a characteristic pKa value.
- Isoelectric point (pI) is the pH at which the net charge on the amino acid is zero. For amino acid with one −COOH and one −NH2, the pI is given as:
Answer to Problem 30QAP
pH = 9.74
Explanation of Solution
The given amino acid is alanine which has the following structure:
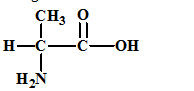
The structures of the respective cationic form (C+), zwitter ion (Z) and the anionic form (A-) are:
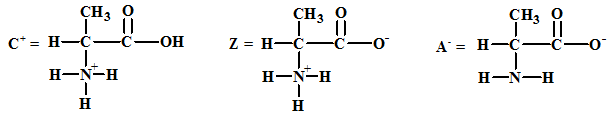
The acid-base equilibria for alanine involving the anion and the zwitter ion forms is:
(c)
Interpretation:
The pH at the isoelectric point needs to be calculated
Concept introduction:
- Amino acids are organic compounds composed of C, H, N and O. The two main functional groups include the amino −NH2 and carboxyl −COOH group in addition to a side chain each with a characteristic pKa value.
- Isoelectric point (pI) is the pH at which the net charge on the amino acid is zero. For amino acid with one −COOH and one −NH2, the pI is given as:
Answer to Problem 30QAP
pH = 6.02
Explanation of Solution
For alanine at the isoelectric point:
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry: Principles and Reactions
- Apatite, Ca5(PO4)3OH, is the mineral in teeth. On a chemical basis explain why drinking milk strengthens young children’s teeth. Sour milk contains lactic acid. Not removing sour milk from the teeth of young children can lead to tooth decay. Use chemical principles to explain why.arrow_forwardIs each of the following substances likely to serve as an oxidantor a reductant: (a) Ce3+(aq), (b) Ca(s), (c) ClO3-(aq),(d) N2O5(g)?arrow_forwardGiven that C6H11COOH has a pKa = 4.8 and C6H11N + H3 has a pKa = 10.7, what pH would you make the water layer to cause both compounds to dissolve in it?arrow_forward
- ) Will the following reaction occur? Explain. (pKa of H2O is 14, pKa of C2H2 is 25, pKa of HCOOH is 3.8)arrow_forward1. Discuss the following roles in the liquid-liquid extraction of dyes. a. Vinegar b. Baking sodaarrow_forwardCalculate the ratio of dihydrogen phosphate to hydrogen phosphate in blood at pH 7.4. The Ka is 6.3 × 10−8.arrow_forward
- explain to me what Isosteric replacements are when talking about strategies for lead molecule modification?arrow_forwardWhat is the net ionic equation for the hydrolysis of NH4Cl?arrow_forwardHow long, in minutes, will it take to produce 54.5 grams of Fe(l) if 80.4 Amps are passed through a solution of FeBr3?arrow_forward
- Calculate the pH of the weak acid HF at equilbrium, if the inital concentration of HF was 0.0340 M. (Ka = 1.45 x 10-5)arrow_forward1b) Suppose you decreased the pH of the biotin solution from 7.0 to 3.0 - what would happen to the ionizable group on a molecule of biotin as the pH shifted from 7.0 to 3.0? Briefly explain why you would expect that to happenarrow_forwardGive the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. Ozokerite Wax Sodium Ascorbate Lactic Acid 90% Titanium Dioxide Magnesium Aluminum Silicate Mineral Oil 50 SUS Tween 80 Zinc Oxide Retinol DMDM HYDANTOINarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

