
To draw: A substituted hydrocarbon with six carbon atoms, hydroxyl groups, single, double, and triple bonds, and amino group.
Explanation of Solution
Introduction:
An organic compound at given conditions can be drawn considering the possible number of bonds an atom can form. For example, C can form maximum of 4 bonds. These can be 4 single bonds, 2 double bonds and 1 triple and 1 single bond. Now, carbon with triple bond can only form an additional single bond thus, triple bond is always at terminal position.
From the name, hydrocarbons are formed from hydrogen and carbon. Now, substituted hydrocarbons means there is a main chain of carbon and hydrogen and different groups are substituted on it.
To determine the structure, first identify types of atoms present in the compound. According to the question, there are 6 carbon atoms, 1 -OH group and 1
All the 6 carbon atoms can be drawn as a main chain. Triple bond is only possible at terminal position and all the other groups can take any position such that valency of all the atoms gets satisfied. There are number of possible structures and one of them is as follows:
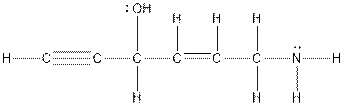
Conclusion:
Thus, the structure of substituted hydrocarbon as per the given information is as follows:
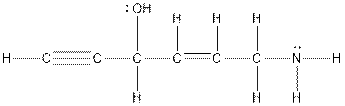
Chapter 23 Solutions
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
Additional Science Textbook Solutions
University Physics with Modern Physics (14th Edition)
University Physics Volume 2
Applied Physics (11th Edition)
Lecture- Tutorials for Introductory Astronomy
An Introduction to Thermal Physics
College Physics: A Strategic Approach (3rd Edition)
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





