
Concept explainers
a)
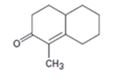
Interpretation:
How to prepare the cyclohexanone shown by combining a Stork enamine reaction with an intramolecular aldol condensation is to be shown.
Concept introduction:
The steps involved in the reaction are i) Reaction of the cyclic
To give:
How to prepare the cyclohexanone shown by combining a Stork enamine reaction with an intramolecular aldol condensation.
b)
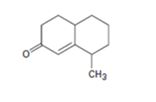
Interpretation:
How to prepare the cyclohexanone shown by combining a Stork enamine reaction with an intramolecular aldol condensation is to be shown.
Concept introduction:
The steps involved in the reaction are i) Reaction of the cyclic ketone with pyrrolidine ii) Michael addition of enamine to cyclohexanone iii) A proton transfer iv) Hydrolysis of the enamine to eliminate the amine v) Abstraction of a proton from the diketone vi) Internal aldol reaction to form the second ring vii) Protonation and dehydration.
To give:
How to prepare the cyclohexanone shown by combining a Stork enamine reaction with an intramolecular aldol condensation.
c)
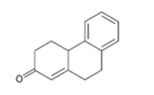
Interpretation:
How to prepare the cyclohexanone shown by combining a Stork enamine reaction with an intramolecular aldol condensation is to be shown.
Concept introduction:
The steps involved in the reaction are i) Reaction of the cyclic ketone with pyrrolidine ii) Michael addition of enamine to cyclohexanone iii) A proton transfer iv) Hydrolysis of the enamine to eliminate the amine v) Abstraction of a proton from the diketone vi) Internal aldol reaction to form the second ring vii) Protonation and dehydration.
To give:
How to prepare the cyclohexanone shown by combining a Stork enamine reaction with an intramolecular aldol condensation.
Trending nowThis is a popular solution!

Chapter 23 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- The Favorskii reaction involves treatment of an -bromo ketone with base to yield a ring-contracted product. For example, reaction of 2-bromocyclohexanone with aqueous NaOH yields cyclopentanecarboxylic acid. Propose a mechanism.arrow_forwardThe enolate derived from diethyl malonate reacts with a variety ofelectrophiles (not just alkyl halides) to form new carbon–carbon bonds.With this in mind, draw the products formed when Na+ −CH(CO2Et)2reacts with each electrophile, followed by treatment with H2O.arrow_forward19.54 Predict the major product(s) from the treatment of acetone with the following: (d) [H], (CH3)2NH, (-H2O) (e) [H], NH2NH2, (-H2O) (f) [H], NH2OH, (-H2O) (g) NaBH4, MeOH (h) RCO3H (i) HCN, KCN (j) EtMgBr followed by H₂O (k) (C6H5)3P=CHCH2CH3 (1) LIAIH, followed by H2Oarrow_forward
- What is the major product generate when p-t-butyl aniline undergoes the following different reactions: a) Nitration b) Bromination c) Friedel-Crafts acylation using isopentanoylchloride in AlCl3 I appreciate the helparrow_forwardWhat is the mechanism of this transformation? Super confused on this mechanism any help is much appreciated Apparently the first step is formation of an iminium cation through the condensation of a primary amine with dihydroxyacetone phosphate.arrow_forwardWhich of A to E shows the likely product obtained from LiAlH4 reduction of the ketoamide shown below?arrow_forward
- The Favorskiireaction involves treatment of an a-bromo ketone with base to yield a ring-contracted product. For example, reaction of 2-bromocyclohexanone with aqueous NaOH yields cyclopentanecarboxylic acid.what is the mechanism of the reaction.arrow_forwardThe intramolecular aldol reaction of 6-oxoheptanal [CH3CO(CH2)4CHO] can yield threecyclic D,E-unsaturated ketones. Give the threeproducts and suggest a mechanism for formingone of the products.arrow_forwardWhich carbonyl compounds do not undergo an aldol reaction when treated with OH in H2O?arrow_forward

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

