
Sulfide ion (S2- ) is formed in wastewater by the action of an aerobic bacteria on organic matter. Sulfide can be readily protonated to form volatile, toxic H2S. In addition to the toxicity and noxious odor, sulfide and H2S cause corrosion problems because they can be easily converted to sulfuric acid when conditions change to aerobic. One common method to determine sulfide is by coulometric titration with generated silver ion.At the generator electrode, the reaction is Ag
(a) A digital chloridometer was used to determine the mass of sulfide in a wastewater sample. The chloridometer reads out directly in ng Cl-.In chloride determinations, the same generator reaction is used,but the titration reaction is Cl- + Ag+
(b) A particular wastewater standard gave a reading of 1689.6 ng Cl-. What total charge in coulombs was required to generate the Ag+ needed to precipitate the sulfide in this standard?
(c) The following results were obtained on 20.00-mL samples containing known amounts of sulfide.17
Each standard was analyzed in triplicate and the mass of chloride recorded. Convert each of the chloride results to mass S2- (ng).
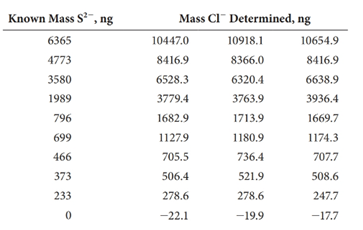
(d) Determine the average mass of S2- (ng), the standard deviation, and the %RSD) of each standard.
(e) Prepare a plot ofthe average mass of S2- determined (ng) versus the actual mass (ng). Determine theslope, the intercept, the standard error, and the R2 value. Comment on the fit of the data to a linear model.
(f) Determine the detection limit (ng) and in parts per million using a k factor of 2 (see Equation 1-12).
(g) An unknown wastewater sample gave an average reading of 893.2 ng Cl. What is the mass of sulfide (ng)? If 20.00 mL of the wastewater sample was introduced into the titration vessel, what is the concentration of S2- n parts per million?
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Principles of Instrumental Analysis
- As part of a soil analysis on a plot of land, a scientist wants to determine the ammonium content using gravimetric analysis with sodium tetraphenylborate, Na+B(C6H5)4−. Unfortunately, the amount of potassium, which also precipitates with sodium tetraphenylborate, is non‑negligible and must be accounted for in the analysis. Assume that all potassium in the soil is present as K2CO3 and all ammonium is present as NH4Cl. A 5.095 g soil sample was dissolved to give 0.500 L of solution. A 150.0 mL aliquot was acidified and excess sodium tetraphenylborate was added to precipitate both K+ and NH4+ ions completely. B(C6H5)4-+K+⟶KB(C6H5)4(s) B(C6H5)4-+NH4+⟶NH4B(C6H5)4(s) The resulting precipitate amounted to 0.269 g. A new 300.0 mL aliquot of the original solution was made alkaline and heated to remove all of the NH4+ as NH3. The resulting solution was then acidified, and excess sodium tetraphenylborate was added to give 0.129 g of precipitate. Find the mass percentages of NH4Cl and…arrow_forwardA BS Medical Technology student was asked to determine the molecular weight of a diprotic acid using acid-base titration. This student performed fourtrials. Firstly, the student used potassium hydrogen phthalate (KHP, 204.22 g/mol) as standard to determine the exact concentration of the NaOHtitrant. The results of the student are summarized in the table below:Note: The stoichiometric relationship of KHP to NaOH is 1:1Standardization Trial 1 Trial 2 Trial 3 Trial 4KHP Mass, g 0.5033 0.5066 0.6989 0.6843Volume NaOH used, mL 24.32 25.61 24.67 24.56After the standardization, the student weighed different amounts of the unknown acid. In a similar fashion, the student performed acid-base titrationusing phenolphthalein as indicator. The results of the molecular weight determination are summarized below:Note: The stoichiometric relationship of Unknown acid to NaOH is 1:2Molecular WeightDeterminationTrial 1 Trial 2 Trial 3 Trial 4Unknown Mass, g 0.1234 0.1034 0.1178 0.1322Volume NaOH used, mL…arrow_forwardA BS Medical Technology student was asked to determine the molecular weight of a diprotic acid using acid-base titration. This student performed fourtrials. Firstly, the student used potassium hydrogen phthalate (KHP, 204.22 g/mol) as standard to determine the exact concentration of the NaOHtitrant. The results of the student are summarized in the table below:Note: The stoichiometric relationship of KHP to NaOH is 1:1Standardization Trial 1 Trial 2 Trial 3 Trial 4KHP Mass, g 0.5033 0.5066 0.6989 0.6843Volume NaOH used, mL 24.32 25.61 24.67 24.56After the standardization, the student weighed different amounts of the unknown acid. In a similar fashion, the student performed acid-base titrationusing phenolphthalein as indicator. The results of the molecular weight determination are summarized below:Note: The stoichiometric relationship of Unknown acid to NaOH is 1:2Molecular WeightDeterminationTrial 1 Trial 2 Trial 3 Trial 4Unknown Mass, g 0.1234 0.1034 0.1178 0.1322Volume NaOH used, mL…arrow_forward
- A BS Medical Technology student was asked to determine the molecular weight of a diprotic acid using acid-base titration. This student performed fourtrials. Firstly, the student used potassium hydrogen phthalate (KHP, 204.22 g/mol) as standard to determine the exact concentration of the NaOHtitrant. The results of the student are summarized in the table below:Note: The stoichiometric relationship of KHP to NaOH is 1:1Standardization Trial 1 Trial 2 Trial 3 Trial 4KHP Mass, g 0.5033 0.5066 0.6989 0.6843Volume NaOH used, mL 24.32 25.61 24.67 24.56After the standardization, the student weighed different amounts of the unknown acid. In a similar fashion, the student performed acid-base titrationusing phenolphthalein as indicator. The results of the molecular weight determination are summarized below:Note: The stoichiometric relationship of Unknown acid to NaOH is 1:2Molecular WeightDeterminationTrial 1 Trial 2 Trial 3 Trial 4Unknown Mass, g 0.1234 0.1034 0.1178 0.1322Volume NaOH used, mL…arrow_forwardSuggest a strategy using selective precipitation (which salts containing precipitating ion would you add and in what order) to purify wastewater contaminated with Ag+, Ba2+, Fe3+ and Na+. The purified water should only contain Na+ which is safe to drink.arrow_forwardSources of Error Determine the relationship between the observed/apparent value (EX) VERSUS that of the true value (ET) for the quantity being sought by writing either <, >, or = on the space provided TOPIC: Measured mass of the precipitate 1. Filter paper was dried prior to filtration. EX _____ ET TOPIC: Standardization of Titrant 2. Distilled water was not equilibrated to room temperature before the preparation of NaOH titrant. EX ______ ET TOPIC: Determination of Molar Concentration of each component (Double Indicator Titration) 3. No blank correction EX ______ ETarrow_forward
- In a redox titration, during the standardization of permanganate, how would the calculated molarity of KMnO4 be affected? 1. The KMnO4 is added too rapidly and too much KMnO4 is added, overshotting the end point. 2. Some of the solid Na2CO4 is spilled on the bench top after weighing but before beginning the titration.arrow_forward123 mL of a 0.352 M Ca(NO3)2 solution is mixed with 251 mL of a 0.254 M solution of NaF at 25ºC. Calculate Qsp for the precipitate formed.arrow_forwardYou have performed an iodimetric titration using a commercial vitamin C tablet. Based on the following information below, calculate the %(w/w) of vitamin C(MM=176.16 g/mol) in the tablet: Mass of tablet dissolved in 250.0 mL: 5.422 g Aliquot volume of sample titrated: 25.00 mL Concentration of KIO3: 0.023 M Final burrette volume: 41.31 mL Initial burrette volume: 8.89 mL Blank volume: 0.14 mLarrow_forward
- CrO42- can be used as an indicator when Br- is titrated with Ag+. What concentration of CrO42- should be used so that Ag2CrO4(s) just starts to form at the equivalence point? If a concentration of 0.0010 M CrO42- is used instead, at what concentration of Br- will Ag2CrO(s) just start to precipitate? Do you think this is likely to introduce significant error? The Ksp for Ag2CrO4(s) is 1.2x10-12.arrow_forwardWhat is the molar solubility of PbBr2 in an aqueous solution containing (1.5x10^-1) M KBr? The Ksp of PbBr2 is 4.6 × 10-6 Note: Your answer is assumed to be reduced to the highest power possible.arrow_forwardA solution of Na3AsO4 is added dropwise to a solution that is 0.0576 M in Cu2+ and 0.000398 M in Ag+.The Ksp of Cu3(AsO4)2 is 7.95e-36.The Ksp of Ag3AsO4 is 1.03e-22.(a) What concentration of AsO43- is necessary to begin precipitation? (Neglect volume changes.)[AsO43-] = ______M.(b) Which cation precipitates first? Cu2+Ag+ (c) What is the concentration of AsO43- when the second cation begins to precipitate?[AsO43-] = _____M.arrow_forward
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning


