
(a)
Interpretation:
The curved arrows for a concerted mechanism for each given reaction are to be drawn.
Concept introduction:
Curved arrows are drawn cyclically to account for the movement of electrons.
Curved arrows in the concerted mechanism are drawn from the number of electrons move in the reaction,
Answer to Problem 24.37P
The curved arrows for a concerted mechanism for each given reaction are:
(i)
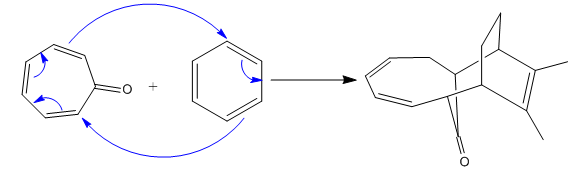
(ii)
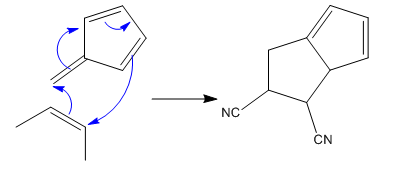
(iii)
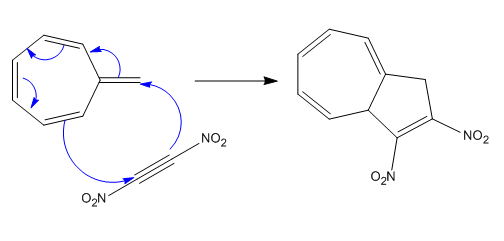
Explanation of Solution
(i)
The given reaction is:
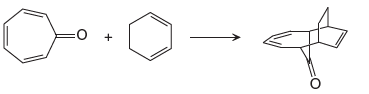
In the above reaction, one reactant has

(ii)
The given reaction is:
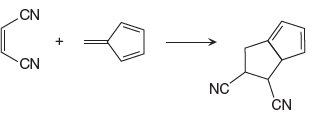
In the above reaction, one reactant has

(iii)
The given reaction is:
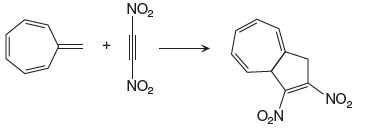
In the above reaction, one reactant has

The curved arrows for a concerted mechanism for the given reaction are drawn using the total number of
(b)
Interpretation:
The transition state formed in each given reaction is to be drawn.
Concept introduction:
Cycloaddition reactions proceeds through the cyclic transition state which represents the breaking and making of bonds.
Answer to Problem 24.37P
The transition state formed in the each given reaction is:
(i)
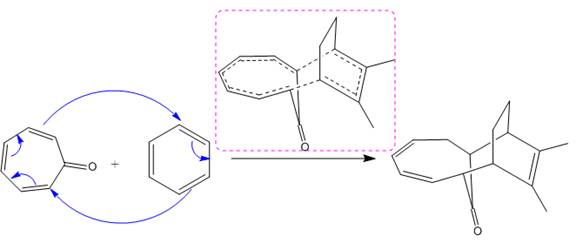
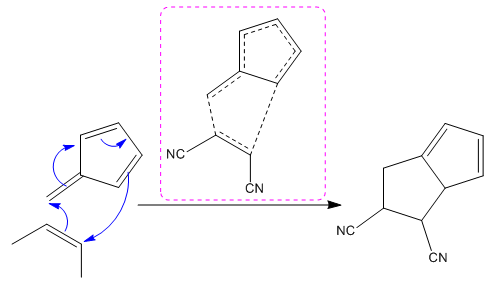
(ii)
(iii)
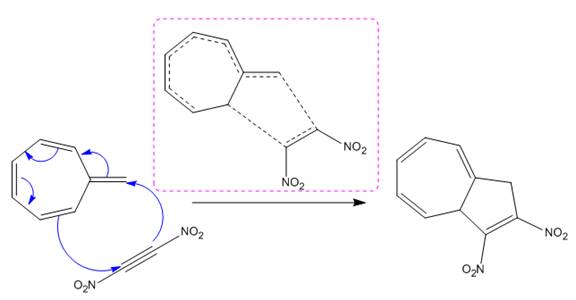
Explanation of Solution
(i)
The given reaction is:

The above cycloaddition reaction proceeds through a cyclic transition state, represents the breaking and making of bond by dash bond.
The transition state had shown the total number of
The transition state formed in the above reaction is marked in the box shown below:
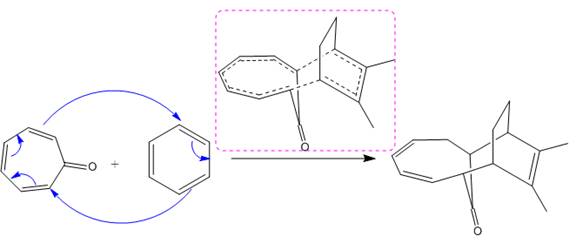
(ii)
The given reaction is:

The above cycloaddition reaction proceeds through a cyclic transition state that represents the breaking and making of the bond by dash bond.
The transition state had shown the total number of
The transition state formed in the above reaction is marked in the box shown below:

(iii)
The given reaction is:

The above cycloaddition reaction proceeds through a cyclic transition state that represents the breaking and making of the bond by dash bond.
The transition state had shown the total number of
The transition state formed in the above reaction is marked in the box show below:
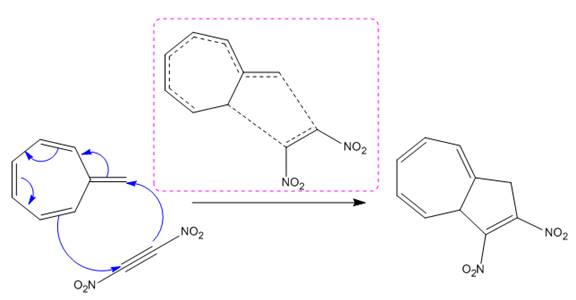
The transition state formed in the each given reaction is drawn using the curved arrows direction with number of
(c)
Interpretation:
The nature of transition state either
Concept introduction:
The transition state aromatic if the number of electrons in that cyclic
Answer to Problem 24.37P
(i) The transition state is aromatic.
(ii) The transition state is antiaromatic
(iii) The transition state is aromatic.
Explanation of Solution
(i)
The concerted mechanism for the given reaction is:
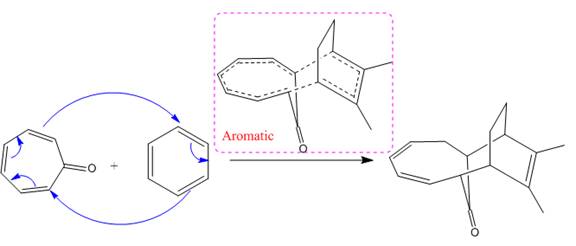
In the above reaction,
(ii)
The concerted mechanism for the given reaction is:
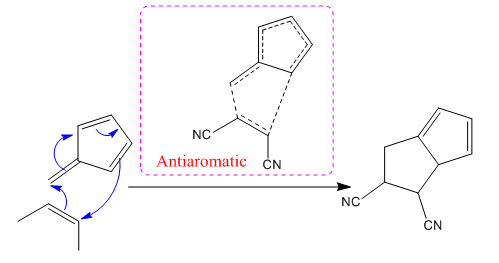
In the above reaction,
(iii)
The concerted mechanism for the given reaction is:
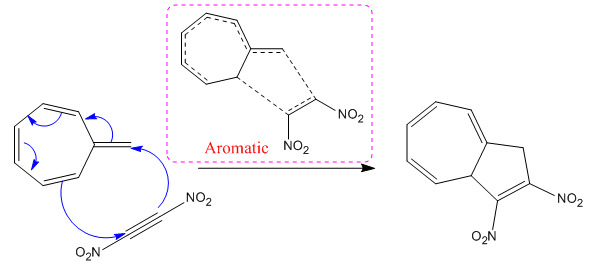
In the above reaction,
The nature of transition state either aromatic or antiaromatic for each reaction is identified from the number of
(d)
Interpretation:
Based on the answer to part (c), whether the reaction is allowed or forbidden under normal conditions is to be identified.
Concept introduction:
The reaction proceeds through aromatic transition state is allowed whereas if the transition state is antiaromatic the reaction is forbidden.
Answer to Problem 24.37P
(i) The given reaction is allowed under normal conditions.
(ii) The given reaction is forbidden under normal conditions.
(iii) The given reaction is allowed under normal conditions.
Explanation of Solution
(i)
The given reaction proceeds through the aromatic transition state, i.e., the number of moving
(ii)
The given reaction proceeds through the antiaromatic transition state, i.e., the number of moving
(iii)
The given reaction proceeds through the aromatic transition state, i.e., the number of moving
Whether the reaction is allowed or forbidden under normal conditions is identified from the nature of the transition state form in the respective reaction.
Want to see more full solutions like this?
Chapter 24 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





