
Concept explainers
(a)
Interpretation:
How to produce the given compound from an
Concept introduction:
In each of these reactions, the
Answer to Problem 24.56P
The starting compound and the reaction condition are given below.
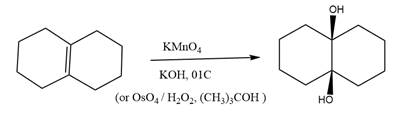
Explanation of Solution
The product from this reaction is a syn 1, 2-
The given reaction is explained as when
(b)
Interpretation:
How to produce the given compound from an alkene or alkyne is to be determined.
Concept introduction:
In each of these reactions, the
Answer to Problem 24.56P
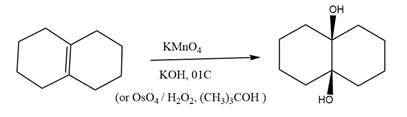
The starting compound and the reaction condition are given below.
Explanation of Solution
The product from this reaction is a syn 1, 2-diol. A
The given reaction is explained as when
(c)
Interpretation:
How to produce the given compound from an alkene or alkyne is to be determined.
Concept introduction:
In each of these reactions, the
Answer to Problem 24.56P
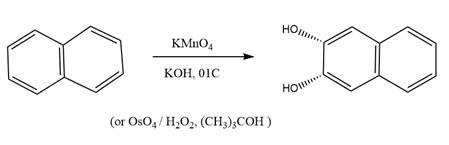
The starting compound and the reaction condition are given below.
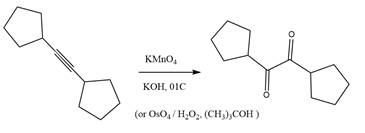
Explanation of Solution
The product from this reaction is a
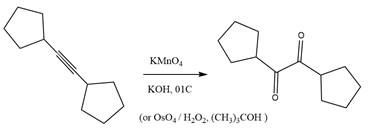
The given reaction is explained as when
(d)
Interpretation:
How to produce the given compound from an alkene or alkyne is to be determined.
Concept introduction:
In each of these reactions, the
Answer to Problem 24.56P
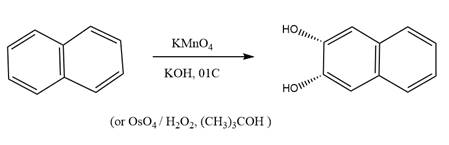
The starting compound and the reaction condition are given below.
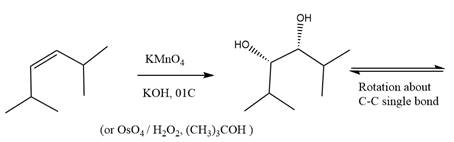
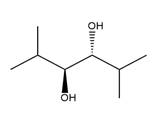
Explanation of Solution
The product from this reaction is a syn 1, 2-diol. A
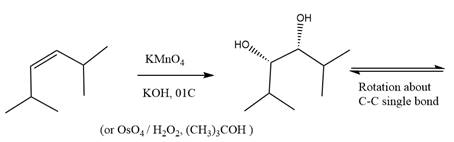
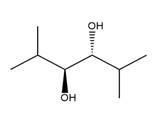
The given reaction is explained as when
Want to see more full solutions like this?
Chapter 24 Solutions
Get Ready for Organic Chemistry
- Show the complete synthesis for the following reaction that forms the following product(s) PLEASE INCLUDE STRUCTURE AND REAGENTarrow_forwardWhat is the skeletal structure of the alkyl halide that forms the following alkene as its only product in an elimination reaction?arrow_forwardDraw the products expected from the addition of bromine to an alkene and to an alkyne.arrow_forward
- Write the product or products that will be formed as a result of the reactions given in each line below.arrow_forwardZaitsev's rule is useful in selecting which carbon adjacent to a carbocation will form the double bond in the alkene product. True or Falsearrow_forwardGive the structure corresponding to following name. (E)-4-ethylhept-3-enearrow_forward
- How Alkynes are prepared by two successive dehydrohalogenationreactions ?arrow_forwardIn the reaction, between the endo and exo isomer that could be produced which is the major product? Explain why your choice is expected to be the major product.arrow_forward5. For each of the following pairs, circle the most stable alkene and provide the name for each of them. X VS. 2.0 VS. VS.arrow_forward
- The addition of water to aldehydes and ketones occurs rapidly, although it is not thermodynamically favored. What would be the product for the reaction above? Hint: Think of the self-ionization of water and the polarity of the carbonyl group.arrow_forwardSelect the bond(s) that is/are formed in the electrophilic addition. H :CI:arrow_forwardExplain the Hydroboration–oxidation two-step reaction sequence that converts an alkene to an alcohol.arrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


