
(a)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).
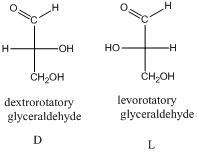
The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To identify: the given carbohydrate (a) is D or L sugar and the configuration of each chiral center.
(b)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To identify: the given carbohydrate (b) is D or L sugar and the configuration of each chiral center.
(c)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To identify: the given carbohydrate (c) is D or L sugar and the configuration of each chiral center.
(d)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To identify: the given carbohydrate (d) is D or L sugar and the configuration of each chiral center.
(e)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To identify: the given carbohydrate (e) is D or L sugar and the configuration of each chiral center.
(f)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To explain: the trend on the configuration of each chiral center in each given carbohydrates.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





