
EBK ORGANIC CHEMISTRY
10th Edition
ISBN: 9781259636875
Author: Carey
Publisher: MCGRAW HILL BOOK COMPANY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 24.9, Problem 10P
Interpretation Introduction
Interpretation:
The most stable conformation of chloromethyl methyl ether is to be sketched.
Concept introduction:
The gauche conformation for structural units of the type
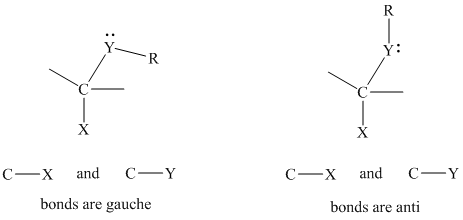
The anomeric effect in this case describes that the heteroatom
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the lowest energy chair conformation of cis-1,3-dimethylcyclohexane, how many axial positions are occupied by methyl groups?
Which do you expect to be the more stable conformation of cis-1,3-dimethylcyclobutane, A or B? Why?
Provide the least stable(highest energy) chair conformation of chlorocyclohexane.
Chapter 24 Solutions
EBK ORGANIC CHEMISTRY
Ch. 24.2 - Prob. 1PCh. 24.3 - Problem 24.2 Which aldotetrose in the structure...Ch. 24.4 - Prob. 3PCh. 24.4 - Prob. 4PCh. 24.6 - Prob. 5PCh. 24.6 - Prob. 6PCh. 24.7 - Prob. 7PCh. 24.8 - Prob. 8PCh. 24.8 - Prob. 9PCh. 24.9 - Prob. 10P
Ch. 24.10 - Prob. 11PCh. 24.10 - Prob. 12PCh. 24.11 - Prob. 13PCh. 24.12 - Prob. 14PCh. 24.14 - Prob. 15PCh. 24.14 - Prob. 16PCh. 24.14 - Prob. 17PCh. 24.14 - Using Mechanism 24.2 as a guide, write a stepwise...Ch. 24.15 - Prob. 19PCh. 24.17 - Prob. 20PCh. 24.17 - Prob. 21PCh. 24.18 - Prob. 22PCh. 24.18 - Prob. 23PCh. 24.18 - Prob. 24PCh. 24.20 - Prob. 25PCh. 24 - Prob. 26PCh. 24 - Prob. 27PCh. 24 - Prob. 28PCh. 24 - Prob. 29PCh. 24 - Prob. 30PCh. 24 - Prob. 31PCh. 24 - Prob. 32PCh. 24 - Prob. 33PCh. 24 - Prob. 34PCh. 24 - Prob. 35PCh. 24 - Methyl glycosides of 2-deoxy sugars have been...Ch. 24 - Prob. 37PCh. 24 - Prob. 38PCh. 24 - Prob. 39PCh. 24 - Prob. 40PCh. 24 - Treatment of d-mannose with methanol in the...Ch. 24 - Prob. 42PCh. 24 - Prob. 43PCh. 24 - Prob. 44PCh. 24 - Prob. 45DSPCh. 24 - Prob. 46DSPCh. 24 - Prob. 47DSPCh. 24 - Prob. 48DSPCh. 24 - Prob. 49DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider 1-bromo-2-methylpropane and draw the following. (a) The staggered conformation(s) of lowest energy (b) The staggered conformation(s) of highest energyarrow_forwardWhich conformation shown below is the most stable conformation of 2-methylbutane?arrow_forwardExplain why the following molecule prefers to adopt an axial conformation for the methyl group.arrow_forward
- Which of these is the most stable conformation?arrow_forwardDraw the most stable conformation of the molecule while taking into account the approximate values of the energies of the following skew interactions:arrow_forwardConsider 1-bromopropane, CH3CH2CH2Br. (a) Which of these is the lowest energy conformation?arrow_forward
- What is the most stable conformation for trans-1,2-dichlorocyclohexane. Describe and explain brieflyarrow_forwardProvide the most stable (lowest energy) chair conformation of methylcyclohexanearrow_forwardwhy is this the chair conformation for the cyclohexane? also is the chair conformation cis or transarrow_forward
- Which is the most stable conformation of the following alcohols? In general, how do I find the most stable conformation?arrow_forwardIn the Diagram below, What is the highest energy conformation of 3-methylpentane when viewed down the 2-3 carbon-carbon bond?arrow_forwardDiscuss and compare two possible chair conformations of cis-1,3-dibromocyclohexane compound. Which conformation is more stable? Why? Explain the reason clearly.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License