
Interpretation:
A structure for atropine (C17H23NO3) is to be proposed from the following observations: i) On basic hydrolysis atropine yields tropic acid C6H5CH(CH2OH)COOH and tropine (C8H15NO). ii) Tropine is an optically inactive alcohol that yields tropidene on dehydration with H2SO4.
Concept introduction:
Esters when hydrolyzed with bases yield alcohols and carboxylic acids. Alcohols when treated with H2SO4 give
To predict:
A structure for atropine (C17H23NO3) from the following observations: i) On basic hydrolysis atropine yields tropic acid C6H5CH(CH2OH)COOH and tropine (C8H15NO). ii) Tropine is an optically inactive alcohol that yields tropidene on dehydration with H2SO4.
Answer:
Structure of atropine is
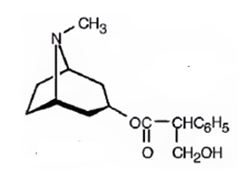
Explanation:
Atropine gives tropic acid and an alcohol, tropine when hydrolyzed in the presence of bases. Hence it must be an ester. Tropine upon dehydration gives tropidene. Hence structure of tropine (optically inactive) can be obtained by adding water to the structure of tropidene.
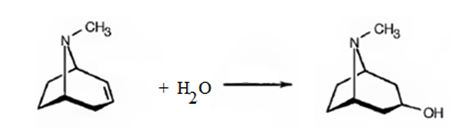
The formation of the ester linkage between the OH of atropine and COOH of tropic acid gives atropine. The reactions can be represented as below.
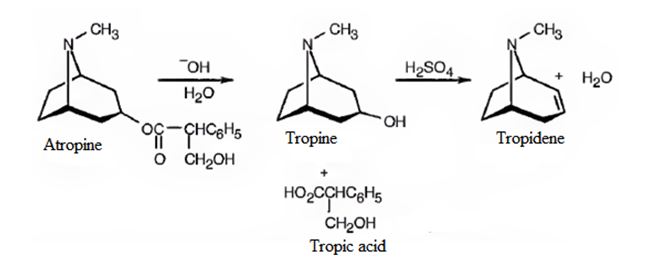
Conclusion:
Structure of atropine is

Trending nowThis is a popular solution!

Chapter 24 Solutions
Organic Chemistry
- As far back as the 16th century, South American Incas chewed the leaves of the coca bush, Erythroxylon coca, to combat fatigue. Chemical studies of Erythroxylon coca by Friedrich Wöhler in 1862 resulted in the discovery of cocaine, C17H21NO4, as the active component. Basic hydrolysis of cocaine leads to methanol, benzoic acid, and another compound called ecgonine, C9H15NO3. Oxidation of ecgonine with CrO3 yields a keto acid that readily loses CO2 on heating, giving tropinone. (a) What is a likely structure for the keto acid? (b) What is a likely structure for ecgonine, neglecting stereochemistry? (c) What is a likely structure for cocaine, neglecting stereochemistry?arrow_forwardThe sex attractant of the common housefly is a hydrocarbon named muscalure, C23H46. On treatment of the muscalure with aqueous acidic KMnO4, two products are obtained, CH3(CH2)12CO2H and CH3(CH2)7CO2H. Propose a structure for the muscalure. Please provide a full explanation with steps when providing the structure as a response, thank you!arrow_forwardBenzoic acid, Ph-COOH (C6H5CO2H), is not soluble in water while it dissolves in ether (diethyl ether), (CH3CH2)2O. Yet upon treatment with sodium hydroxide, benzoic acid turns hydrophilic and dissolves in water. Provide chemical explanation of this observation.arrow_forward
- Write the chemical equation for the acid dissociation of acetaminophen, C8H9O2N. Write the Ka expression for the acid dissociation of acetaminophen.arrow_forwardWrite the equilibrium-constant expressions and obtainnumerical values for each constant in. (a) the basic dissociation of aniline, C6H5NH2. (b) the acidic dissociation of hypochlorous acid,HClO. (c) the acidic dissociation of methyl ammoniumhydrochloride, CH3NH3Cl. (d) the basic dissociation of NaNO2. (e) the dissociation of H3AsO3to H3O+and AsO33-. (f) the reaction of C2O42-with H2O to give H2C2O4and OH-. show solutionarrow_forward8 In Chapter 22, we will discuss a class of compounds called amino acids, so named because they contain both an amino group and a carboxyl group. Following is a structural formula for the amino acid alanine. What would you expect to be the major form of alanine present in aqueous solution (a) at pH 2.0, (b) at pH 5—6, and (c) at pH 11.0? Explain.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


