
(a)
Interpretation:
A curved-arrow mechanism for the interconversion of
Concept introduction:
The interconversion of
Answer to Problem 25.35AP
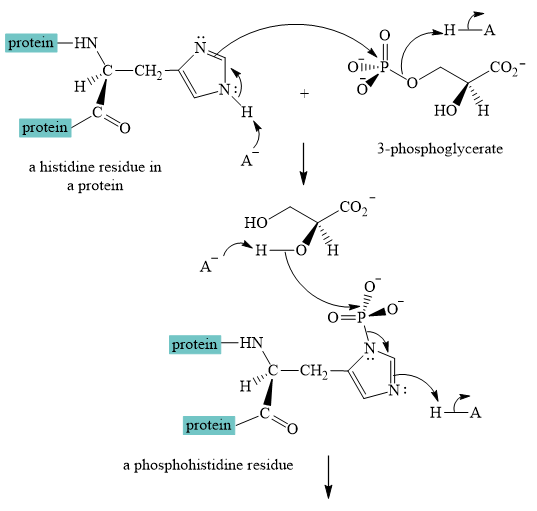
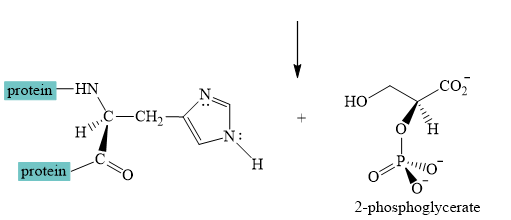
Explanation of Solution
The interconversion equation of
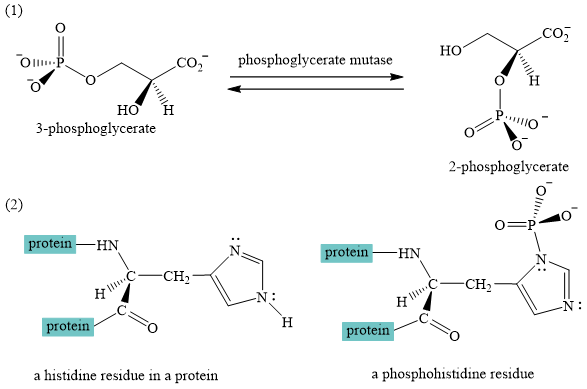
A curved-arrow mechanism for the interconversion of
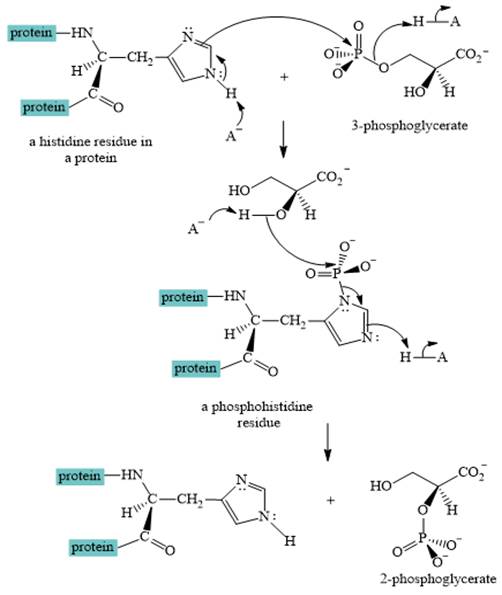
The nitrogen of histidine residue acts as a nucleophile after loss of proton by a base
The two step curved arrow mechanism for the interconversion of
(b)
Interpretation:
If the isotopically chiral
Concept introduction:
The enzyme catalyzed substitution reaction of phosphate ester derivate at phosphate proceeds through
Answer to Problem 25.35AP
If enantiomerically pure
Explanation of Solution
The enzyme catalyzed substitution reaction of phosphate ester derivate at phosphate proceeds through inversion of stereochemistry. The substitution occurs twice with double inversion of the product formed with retention of configuration. Thus, if enantiomerically pure
The interconversion of
Want to see more full solutions like this?
Chapter 25 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Somatostatin is a tetradecapeptide of the hypothalamus that inhibits the release of pituitary growth hormone. Its amino acid sequence has been determined by a combination of Edman degradations and enzymic hydrolysis experiments. On the basis of the following data, deduce the primary structure of somatostatin: 1. Edman degradation gave PTH-Ala. 2. Selective hydrolysis gave peptides having the following indicated sequences: Phe-Trp Thr-Ser-Cys Lys-Thr-Phe Thr-Phe-Thr-Ser-Cys Asn-Phe-Phe-Trp-Lys Ala-Gly-Cys-Lys-Asn-Phe 3. Somatostatin has a disulfide bridge.arrow_forwardIllustrate a glycosidic, peptide and a phospho-diester bond.arrow_forwardAssume that for your Biochemistry practical, you were asked to synthesise D-Galactose. You went to chemical storage room to take some D-lyxose to use as the starting material. But there you found that labels had fallen off from the bottles containing D-lyxose and D-xylose. How could you determine which bottle contains D- lyxose?arrow_forward
- The amino acid (S)-alanine has the physical characteristics listed under the structure. How does the melting point of a racemic mixture of (R)- and (S)-alanine compare to the melting point of (S)-alanine?arrow_forwardEsterase is an enzyme that catalyzes the hydrolysis of esters. It hydrolyzes esters of L-amino acids more rapidly than esters of d-amino acids. How can this enzyme be used to separate a racemic mixture of amino acids?arrow_forwardSuggest how you would separate the free l-amino acid from its acylated d enantiomerarrow_forward
- Is gentiobiose a reducing sugar? Does it mutarotate? Explain your reasoningarrow_forwardIn a paragraph form provide the experimental procedure of the reaction of oxazetidine-containing peptides and α-ketoacid that will result in protein that contain native serine residuesarrow_forwardWhich separation technique is the most specific and offers the highest protein purification possible?arrow_forward
- Another strategy used to resolve amino acids involves converting the carboxy group to an ester and then using a chiral carboxylic acid to carry out an acid–base reaction at the free amino group. Using a racemic mixture of alanine enantiomers and (R)-mandelic acid as resolving agent, write out the steps showing how a resolution process would occur.arrow_forwardIn the experiments of Barrick et al. (as shown), it was observed thatreplacement of histidine by a noncovalently bonded imidazole not onlyreduced cooperativity but also increased the oxygen affinity of the hemoglobin. Suggest an explanation.arrow_forwardGiven the following peptide SEPIMAPVEYPK(a) Estimate the net charge at pH 7 and at pH 12. Assume the pKa valuesgiven in as shown. (b) How many peptides would result if this peptide were treated with(1) cyanogen bromide, (2) trypsin, or (3) chymotrypsin?(c) Suggest a method for separating the peptides produced by chymotrypsintreatment.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

