
Concept explainers
Interpretation:
The isoprene unit in
Concept introduction:
The terpenes are the repeating isoprene units.
The isoprene unit is nothing but the carbon skeleton of isoprene without double bonds.
The isoprene molecule has head and tail. The structure of isoprene
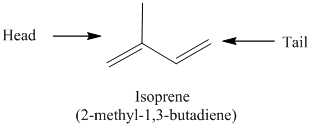
The isoprene units are together linked in head-to-tail configuration. The tail of the first isoprene molecule joins with the head of the second isoprene molecule.
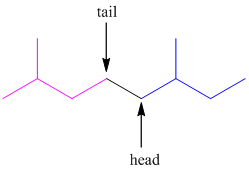
In higher terpenes, tail-to-tail linkage can also occur. The tail of the first isoprene molecule joins with the tail of the second isoprene molecule.
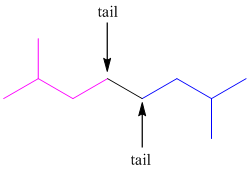
As the terpenes forms by isomerization of isoprene unit, both the double bonds of isoprene may not be present in the structure of terpenes.
On the basis of number of isoprene units
Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
Organic Chemistry - Standalone book
- Fill in the empty blanks and spaces in the table. The structures of d-galactose and a-D-galactopyranose are in the next picture for reference.arrow_forwardIs the attached compound not composed of isoprene units? Locate the isoprene units in each compound that contains them.arrow_forwardEncircle the functional groups found in Betacarotene and explain each of their purposes in the compound thoroughlyarrow_forward
- What is the structure and components/composition of Cellulose in Fischer and Haworth form? Please explainarrow_forwardis Isoprene ionic or covalent? Why?arrow_forwardIt is well known that humans are able to digest amylose, but not cellulose. What structural features might be responsible this observation?arrow_forward
- How many stereoisomers are possible for this structure? How many chiral carbons are in the structure?arrow_forwardIn 1935, J. Bredt, a German chemist, proposed that a bicycloalkene could not have a double bond at a bridgehead carbon unless one of the rings contains at least eight carbons. This is known as Bredt’s rule. Explain why there cannot be a double bond at this position.arrow_forwardName all the functional groups of highly branched isoprenoids. Point out chiral centers and/or cis/trans double bonds if there are any and explain the significance of these structural components.arrow_forward
- I have already drawn helicene as an example but I am stuck on thinking of two other compounds. I know that an asymmetrically substituted carbon atom has 4 different substituents attached to the carbon. But, I am confused about finding other structures that are still chiral but do not have an asymmetrically substituted carbon atom.arrow_forwardWhich isoprene units are connected in a head-to-tail fashion?arrow_forwardWhat are corresponding geometry for structure with steric number 8?arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole  Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning



