
Concept explainers
a)
Interpretation:
The configurational stereochemistry of the molecules to be determined.
Answer to Problem 26VC
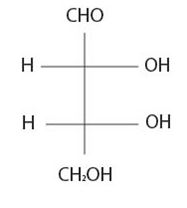
The Fischer projection follows as
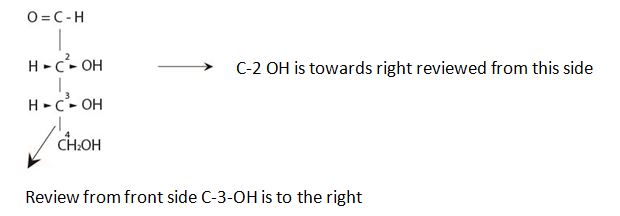
It is D sugar the molecule is a retrose. Elclose or eldotetrose, is 4-carbon elclose.
Explanation of Solution
Concept strategy: The Fischer projection of the given monosaccharide is drawn vertically, by rotating the molecule anticlockwise 90° so that the carbonyl
Review from front side C-3-OH is to the right By convention the molecule has the C-3 hyduxyl at the right. So it is D sugar the molecule is a retrose. Elclose or eldotetrose, is 4-carbon elclose.
Based on the Fischer projection formula for the given sugars it is a B-D-glucopyranose monosaccharide.
b)
Interpretation:
The configurational stereochemistry of the molecules to be determined.
Answer to Problem 26VC
The given model is the cyclic structures of an aldohexose in six membered pyranose form.
Strategy: We redraw the model as
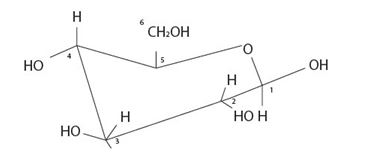
Explanation of Solution
By convention, the terminal -CH2OH group is on the top of the chair Pyranose structure. Thus it is a D sugar. The molecule is an aldohexose is B-D-glucopyranose, all the –OH groups are equatorial (and more stable due to minimum repulsion) conformation.
Based on the Fischer projection formula for the given sugars it is a B-D-glucopyranose monosaccharide.
Want to see more full solutions like this?
Chapter 25 Solutions
ORGANIC CHEM.(LL)-W/OWL V2 >CUSTOM<
- trehalose is a disacharide that can be obtained from fungi sea uchins and insects. acid hydrolysis of trehalose yields only D-glucose. trehalose is hydrolysed by a-glucosidase but not b-glucosidase.methylation of trhalose followed by hydrolysis yield two molar equivalents of 2-3-4-6 -tetra-O-methyl-D-glucopyranose. deduce the structure of the trehalose using the experimental dataarrow_forwardTreatment with NaBH 4 converts aldose U into an optically inactive (meso) alditol V. Ruff degradation ofU gives W, whose alditol is optically inactive. Ruff degradation of W forms D-glyceraldehyde, thesimplest aldose. Upon Kiliani-Fischer synthesis, U is converted to two aldoses, X and Y. X is oxidized toan optically active aldaric acid Z. Y is oxidized to an optically inactive aldaric acid. Draw the structuresof D-glyceraldehyde, V, W, X, Y, and Z. Structure of compound U is shown below.arrow_forwardAn oligosaccharide isolated from an organism is found tocontain two glucose residues and one galactose residue.Exhaustive methylation followed by hydrolysis producedtwo glucoses with methoxy groups at positions 2, 3,and 6 and galactose with methoxy groups at positions2, 3, 4, and 6. What is the structure of the originaloligosaccharide?arrow_forward
- 1. Trehalose is a disaccharide that can be obtained from fungi, sea urchins and insects. Acid hydrolysis of trehalose yields only D-glucose. Trehalose is hydrolysed by α-glucosidase and not by β-glucosidase enzymes. Methylation of trehalose followed by hydrolysis yields two molar equivalents of 2,3,4,6-tetra-O-methyl-D-glucopyranose.From the following experimental data, deduce the structure of trehalose.What will be the effect of trehalose on Fehling’s solution? 2.Suggest a test you will use to show that a given food substance contains proteinarrow_forwardMonosaccharide Structure and Stereochemistry For D-arabinose: Draw an epimer at C3.arrow_forwardTreatment of D-glucose with NaBH4 gives an alditol A. What Laldohexosealso yields A when treated with NaBH4?arrow_forward
